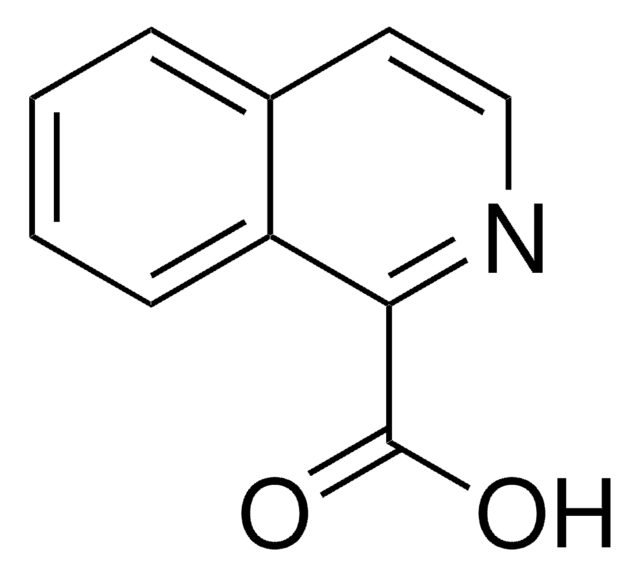
추천 제품
분석
97%
mp
254-255 °C (lit.)
작용기
carboxylic acid
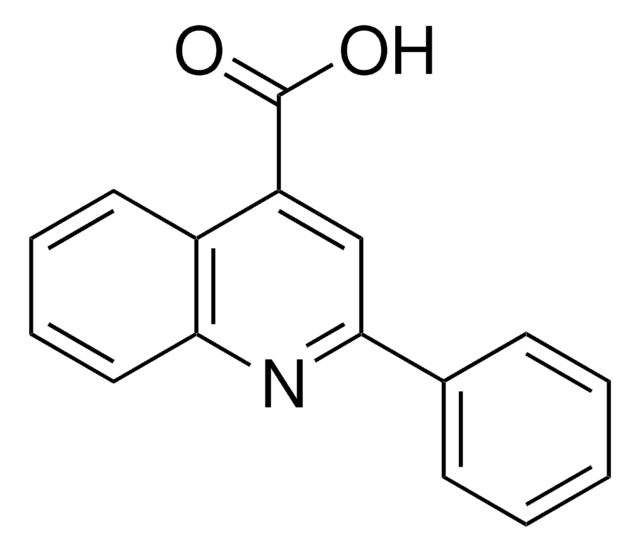
SMILES string
OC(=O)c1ccnc2ccccc12
InChI
1S/C10H7NO2/c12-10(13)8-5-6-11-9-4-2-1-3-7(8)9/h1-6H,(H,12,13)
InChI key
VQMSRUREDGBWKT-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
4-Quinolinecarboxylic acid was used in the coupling reaction with diamine linker. A 4-quinolinecarboxylic acid analogue, brequinar sodium was used to inhibit dihydroorotate dehydrogenase and the de novo biosynthesis of pyrimidine.
생화학적/생리학적 작용
4-Quinolinecarboxylic acid showed anti-tumor activity against L1210 leukemia and B16 melanoma.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
D L Dexter et al.
Cancer research, 45(11 Pt 1), 5563-5568 (1985-11-01)
A novel, substituted 4-quinolinecarboxylic acid (NSC 339768) demonstrated antitumor activity against L1210 leukemia and B16 melanoma in the National Cancer Institute's Developmental Therapeutics Program. An extensive analogue synthesis program was initiated; over 200 derivatives were synthesized and tested for anticancer
[A case of Gaucher's disease treated with hydroxyphenylcinchoninic acid].
P DANIEL MARTINEZ et al.
Boletin medico del Hospital Infantil de Mexico, 8(2), 189-194 (1951-04-01)
A J Dobson et al.
Acta crystallographica. Section C, Crystal structure communications, 55 ( Pt 7), 1192-1195 (1999-08-13)
The previously undescribed title substance, C10H7NO2.-3H2O, crystallized in the centrosymmetric space group P1 with one zwitterionic organic molecule and three water molecules in the asymmetric unit. One N-H...O and six O-H...O hydrogen bonds are present in this structure, with donor-acceptor
Murugesan Dinakaran et al.
Medicinal chemistry (Shariqah (United Arab Emirates)), 4(5), 482-491 (2008-09-11)
Thirty four novel 7-fluoro/nitro-1,2-dihydro-5-oxo-8-(sub)-5H-thiazolo[3,2-a]quinoline-4-carboxylic acids were synthesized from 2,4-dichlorobenzoic acid and 2,4-dichloro-5-fluoroacetophenone by multi step reaction, evaluated for in vitro and in vivo antimycobacterial activities against Mycobacterium tuberculosis H37Rv (MTB), multi-drug resistant Mycobacterium tuberculosis (MDR-TB) and Mycobacterium smegmatis (MC2) and
He Huang et al.
The Journal of organic chemistry, 74(15), 5476-5480 (2009-07-04)
We developed a simple and convenient copper-catalyzed method for the synthesis of quinoline-2-carboxylate derivatives through sequential intermolecular addition of alkynes onto imines and subsequent intramolecular ring closure by arylation. The efficiency of this system allowed the reactions to be carried
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.