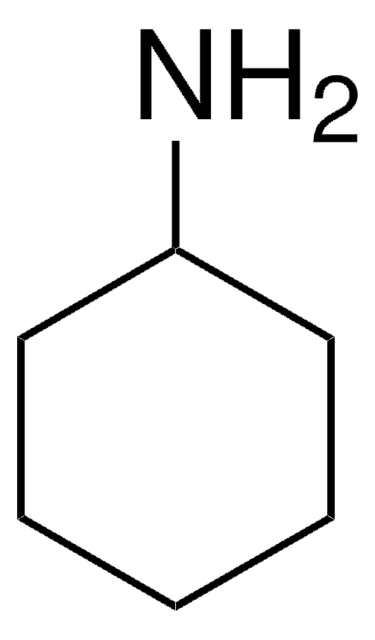
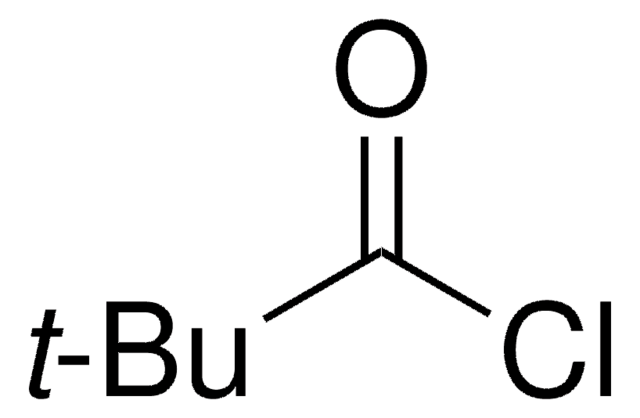
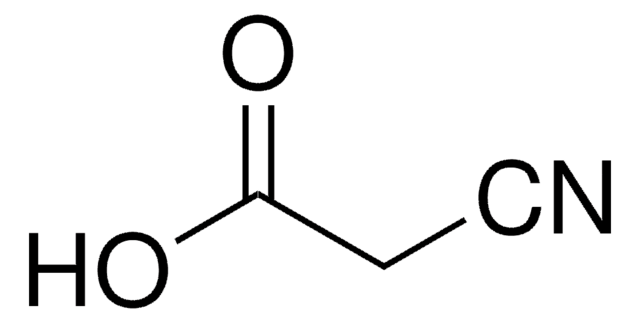
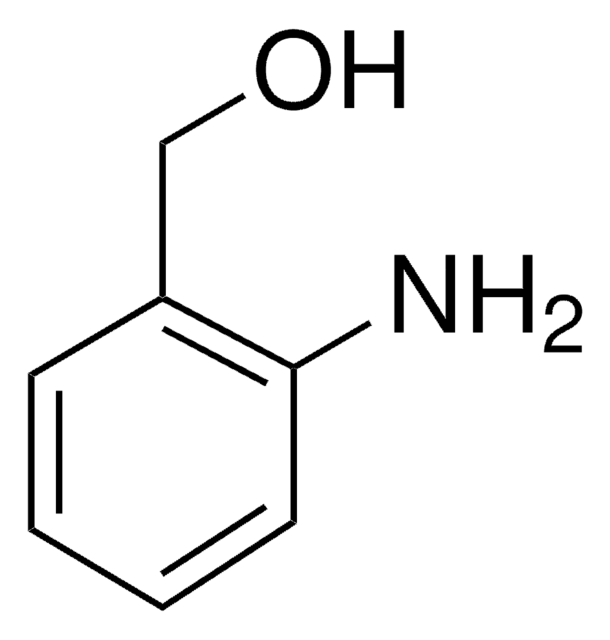
추천 제품
vapor density
>1 (vs air)
vapor pressure
<0.01 mmHg ( 20 °C)
분석
98%
양식
liquid
refractive index
n20/D 1.578 (lit.)
bp
278-282 °C/760 mmHg (lit.)
density
1.094 g/mL at 25 °C (lit.)
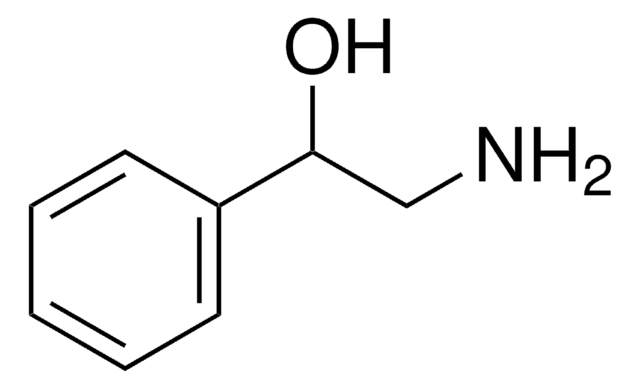
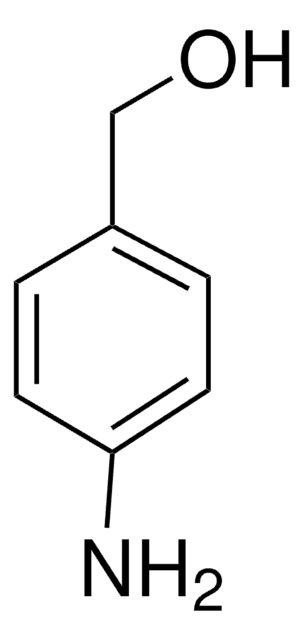
SMILES string
OCCNc1ccccc1
InChI
1S/C8H11NO/c10-7-6-9-8-4-2-1-3-5-8/h1-5,9-10H,6-7H2
InChI key
MWGATWIBSKHFMR-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
N-(2-Hydroxyethyl)aniline was employed as substrate for human olfactory UDP-glucuronosyltransferase.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 2 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Dam. 1 - STOT RE 2 - STOT SE 1
표적 기관
Blood, Blood,hematopoietic system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Mercedes Amat et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(28), 7724-7732 (2011-06-15)
Phenylglycinol-derived, unsaturated oxazolopiperidone lactams are extremely useful building blocks that undergo stereoselective conjugate addition reactions with organocuprates, enolates, and sulfur-stabilized anions, allowing the stereocontrolled introduction of substituents at the piperidine 4-position. The factors governing the exo- or endo-facial selectivity are
Alok K Awasthi et al.
The Journal of organic chemistry, 70(14), 5387-5397 (2005-07-02)
[reaction: see text] A practical, large-scale synthesis of a beta-amino ester 1 was developed. A chiral imine derived from (S)-phenylglycinol and 3-trimethylsilylpropanal was coupled with the Reformatsky reagent 3 with high diastereoselectivity (de > 98%) to give (SS)-4a as the
Katarina Babić et al.
Journal of chromatography. A, 1142(1), 84-92 (2006-10-19)
The performance of extractant impregnated resin (EIR) technology for chiral separation of amino-alcohols has been investigated. Phenylglycinol was selected as an archetype model enantiomer and azophenolic crown ether was used as a versatile enantioselective extractant. 1-Phenyloctane was selected as a
Mercedes Amat et al.
Chemical communications (Cambridge, England), (20)(20), 2935-2937 (2009-05-14)
The first total synthesis of (-)-16-episilicine has been completed from a phenylglycinol-derived bicyclic lactam, the key steps being stereoselective conjugate addition and alkylation reactions, and a ring-closing metathesis.
Marco Santarem et al.
The Journal of organic chemistry, 73(16), 6466-6469 (2008-07-22)
An efficient formal total synthesis of (+)-gephyrotoxin is described. The key step of our strategy relies on the diastereoselective reduction of a chiral pyrrolidine beta-enamino ester obtained by condensation of ( S)-phenylglycinol on a protected 8-hydroxy-3,6-dioxooctanoate.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.