모든 사진(1)
About This Item
Linear Formula:
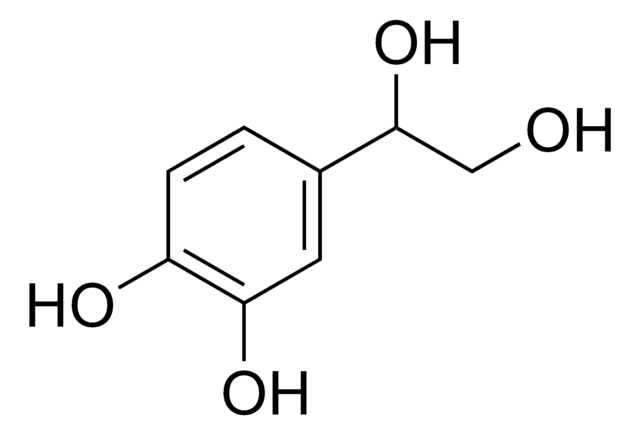
(HO)2C6H3CH(OH)CO2H
CAS Number:
Molecular Weight:
184.15
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
95%
mp
136-137 °C (dec.) (lit.)
SMILES string
OC(C(O)=O)c1ccc(O)c(O)c1
InChI
1S/C8H8O5/c9-5-2-1-4(3-6(5)10)7(11)8(12)13/h1-3,7,9-11H,(H,12,13)
InChI key
RGHMISIYKIHAJW-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
Metabolite of norepinephrine.
애플리케이션
DL-3,4-Dihydroxymandelic acid was used in the simultaneous analysis of 4-hydroxy-3-methoxymandelic acid and 4-hydroxy- 3-methoxyphenylacetic acid in urine. It was also used to study the changes in body temperature.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
W X Dong et al.
Journal of the autonomic nervous system, 44(2-3), 109-117 (1993-08-01)
Pre-synaptic endings of the sympathetic nervous fibers control the metabolism of catecholamines, particularly inactivating norepinephrine after its neuronal recapture. The present study was carried out to investigate this segment of the metabolism of catecholamines through measurements of DHPG, DOMA and
S J Ley et al.
Research in veterinary science, 61(2), 172-173 (1996-09-01)
The threshold response to a mechanical nociceptive stimulus was significantly lower on the lame hind limb of lame cows than on the same limb of sound cows. There were no significant differences between the concentrations of cortisol, noradrenaline, adrenaline or
K E O'Connor et al.
Journal of bacteriology, 183(3), 928-933 (2001-02-24)
Pseudomonas putida F6 was found to metabolize p-hydroxyphenylacetic acid through 3,4-dihydroxyphenylacetic acid, 3,4-dihydroxymandelic acid, and 3,4-dihydroxybenzaldehyde. Cell extracts of P. putida F6 catalyze the NAD(P)H-independent hydroxylation of p-hydroxyphenylacetic acid to 3,4-dihydroxyphenylacetic acid which is further oxidized to 3,4-dihydroxymandelic acid. Oxidation
T R Kingsley et al.
Journal of gerontology, 46(4), B135-B141 (1991-07-01)
Adrenal catecholamines (CA) were measured in 6-, 18-, and 30-mo Lobund-Wistar rats (LWR) maintained under germ-free or conventional conditions and fed either ad libitum or a restricted (70% of adult ad libitum) diet. Levels of dopamine (DA), norepinephrine (NE), epinephrine
T H Czapla et al.
Biochimica et biophysica acta, 1077(3), 400-406 (1991-04-29)
Cyclic voltammetric and chronoamperometric data are consistent with a process in which 3,4-dihydroxymandelic acid (DOMA) is oxidized initially in a two-electron step to its corresponding o-benzoquinone. This species is unstable and undergoes the rate-determining loss of CO2 (k = 1.6
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.