추천 제품
Quality Level
분석
98%
양식
solid
mp
141-143 °C (lit.)
solubility
ethanol: soluble 10 mg/mL, clear, light yellow to yellow
작용기
ketone
SMILES string
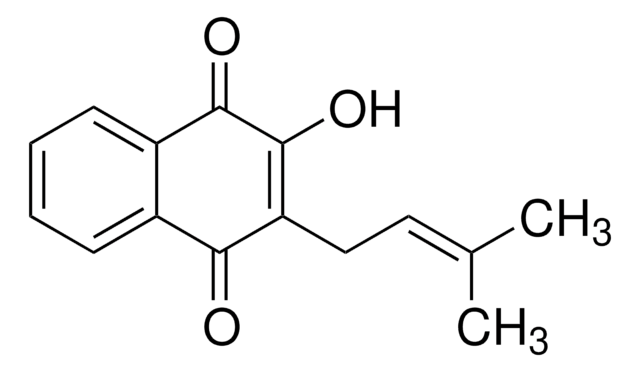
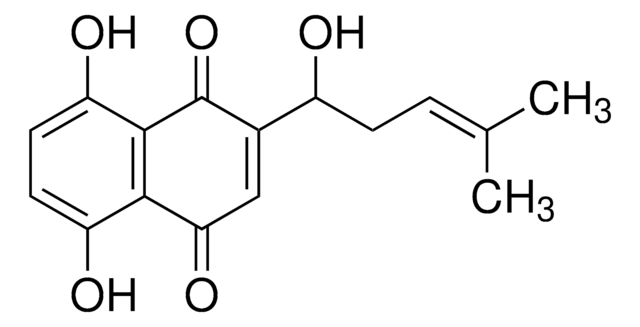
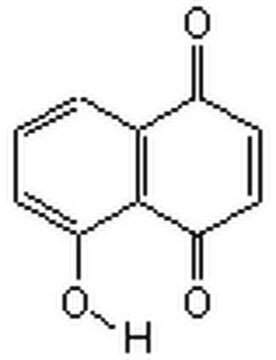
C\C(C)=C\CC1=C(O)C(=O)c2ccccc2C1=O
InChI
1S/C15H14O3/c1-9(2)7-8-12-13(16)10-5-3-4-6-11(10)14(17)15(12)18/h3-7,18H,8H2,1-2H3
InChI key
CIEYTVIYYGTCCI-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Lapachol is natural naphthoquinone compound derived from Bignoniaceae (Tabebuia sp.).
애플리케이션
Lapachol was used in the synthesis of the lapachol metal complexes.
생화학적/생리학적 작용
Lapachol has antimicrobial properties against many pathogens. It has anti-inflammatory, analgesic and antibiotic properties. It is inhibitor of epithelial tumors in Drosophila melanogaster heterozygote.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
이미 열람한 고객
Ricardo Vessecchi et al.
Journal of mass spectrometry : JMS, 47(12), 1648-1659 (2013-01-03)
In order to understand the influence of alkyl side chains on the gas-phase reactivity of 1,4-naphthoquinone derivatives, some 2-hydroxy-1,4-naphthoquinone derivatives have been prepared and studied by electrospray ionization tandem mass spectrometry in combination with computational quantum chemistry calculations. Protonation and
Cristian Salas et al.
Bioorganic & medicinal chemistry, 16(2), 668-674 (2007-11-22)
Derivatives of natural quinones with biological activities, such as lapachol, alpha- and beta-lapachones, have been synthesized and their trypanocidal activity evaluated in vitro in Trypanosoma cruzi cells. All tested compounds inhibited epimastigote growth and trypomastigote viability. Several compounds showed similar
Eduardo J S Salustiano et al.
Investigational new drugs, 28(2), 139-144 (2009-03-04)
The pentacyclic 1,4-naphthoquinones 1a-d were cytotoxic (IC(50) approximately 2-7 microM) to human leukemic cell lines K562 (oxidative stress-resistant), Lucena-1 (MDR phenotype) and Daudi. Fresh leukemic cells obtained from patients, some with the MDR phenotype, were also sensitive to these compounds.
Lu Bai et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 944, 128-135 (2013-12-10)
Lapachol is a natural naphthoquinone compound derived from Bignoniaceae (Tabebuia sp.) that possesses a range of significant biological activities. Nine phase I and four phase II metabolites of lapachol in rat bile were firstly elucidated and identified using a sensitive
Renato A S Oliveira et al.
International immunopharmacology, 10(11), 1463-1473 (2010-09-15)
The present study reports the anti-mycobacterial activity of 2-hydroxy-3-(3-methyl-2-butenyl)-1,4-naphthoquinone (lapachol) as well as its influence on macrophage functions. Lapachol (L) did not induce apoptosis/necrosis of THP-1 macrophages at ≤32 μg/mL. Mycobacterium avium liquid growth was arrested by ≥32 μg/mL and
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.