모든 사진(3)
About This Item
실험식(Hill 표기법):
C14H10
CAS Number:
Molecular Weight:
178.23
색상 지수 번호:
10790
Beilstein:
1905429
EC Number:
MDL number:
UNSPSC 코드:
12352100
eCl@ss:
39011608
PubChem Substance ID:
NACRES:
NA.22
추천 제품
vapor density
6.15 (vs air)
Quality Level
vapor pressure
1 mmHg ( 145 °C)
제품 라인
ReagentPlus®
분석
99%
양식
powder or flakes
autoignition temp.
1004 °F
색상
blue-violet fluorescence
bp
340 °C (lit.)
mp
210-215 °C (lit.)
solubility
toluene: soluble 20 mg/mL, clear, colorless to faintly yellow
alcohols: soluble
benzene: soluble
chloroform: soluble
hydronaphthalenes: soluble
supercritical carbon dioxide: soluble
SMILES string
c1ccc2cc3ccccc3cc2c1
InChI
1S/C14H10/c1-2-6-12-10-14-8-4-3-7-13(14)9-11(12)5-1/h1-10H
InChI key
MWPLVEDNUUSJAV-UHFFFAOYSA-N
유전자 정보
human ... CYP1A2(1544)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Anthracene derivatives are useful in preparing stable blue-emitting organic electroluminescence devices. It is oxidized to anthracene cis-1,2-dihydrodiol by Mycobacterium sp. strain PYR-12. It forms bis-adducts by [4+2]-cycloaddition reaction with [5,6]-fullerene C60.
애플리케이션
Anthracene has been shown to be soluble in a variety of binary and ternary mixtures of cyclohexanone, ethyl acetate, and methanol .
Anthracene was used to study the photodimerization reaction of anthracene in supercritical CO2.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
249.8 °F - closed cup
Flash Point (°C)
121.0 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Christopher E. Bunker et al.
The Journal of organic chemistry, 62(21), 7324-7329 (2001-10-24)
The photodimerization reaction of anthracene in supercritical CO(2) was studied systematically at different CO(2) densities. Unlike in normal liquid solvents, the reaction in supercritical CO(2) is significant even at anthracene concentrations as low as a few micromolar. At comparable anthracene
J D Moody et al.
Applied and environmental microbiology, 67(4), 1476-1483 (2001-04-03)
Cultures of Mycobacterium sp. strain PYR-1 were dosed with anthracene or phenanthrene and after 14 days of incubation had degraded 92 and 90% of the added anthracene and phenanthrene, respectively. The metabolites were extracted and identified by UV-visible light absorption
Anthracene derivatives for stable blue-emitting organic electroluminescence devices.
Jianmin S and Tang CW.
Applied Physics Letters, 80(17), 3201-3203 (2002)
The bis-adducts of the [5, 6]-fullerene C60 and anthracene.
Duarte-Ruiz A, et al.
Tetrahedron, 57(17), 3709-3714 (2001)
Hyunjung Lee et al.
Inorganic chemistry, 51(20), 10904-10915 (2012-09-26)
The tendency of a Hg(II) ion to strongly quench fluorescence of potential fluorescent sensors is explored. Fluorescence measurements show the expected order of the chelation-enhanced fluorescence (CHEF) effect of Zn(II) > Cd(II) > Hg(II) ~ Cu(II), which is interpreted as
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.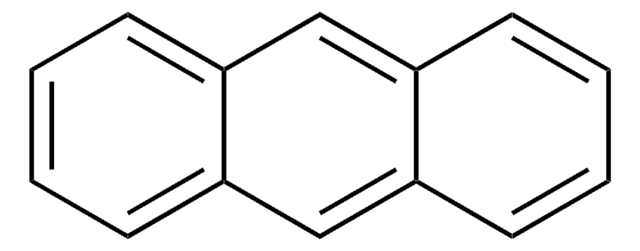
![Benz[b]anthracene 98%](/deepweb/assets/sigmaaldrich/product/structures/197/885/3a015625-5e09-4f15-8b17-4cc285304fc7/640/3a015625-5e09-4f15-8b17-4cc285304fc7.png)