139416
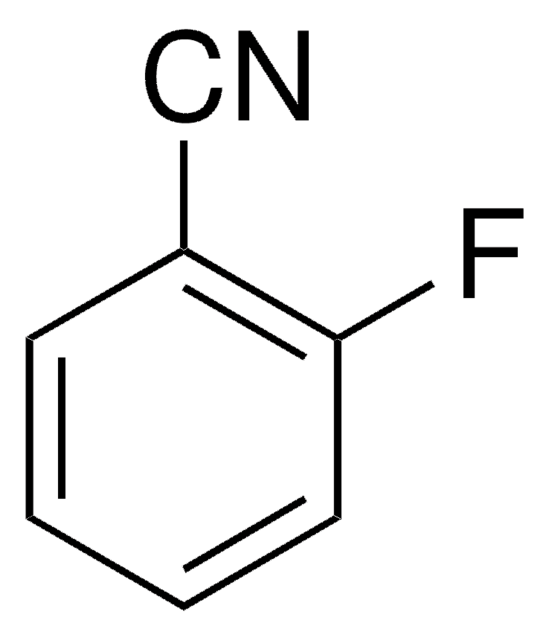
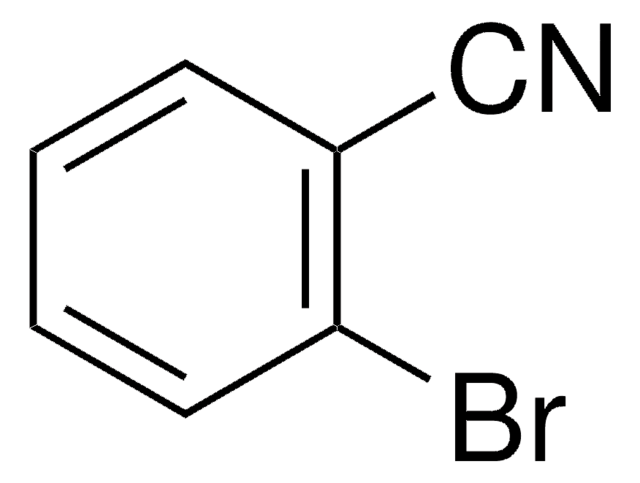
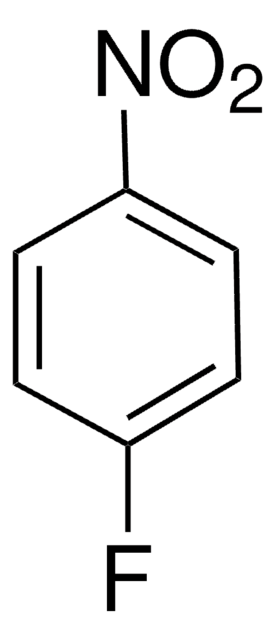
4-Fluorobenzonitrile
99%
동의어(들):
1-Cyano-4-fluorobenzene, 4-Cyanofluorobenzene, 4-Fluorobenzonitrile, 4-Fluorocyanobenzene, Para-fluorobenzonitrile, p-Cyanofluorobenzene, p-Fluorobenzonitrile, p-Fluorophenyl cyanide
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
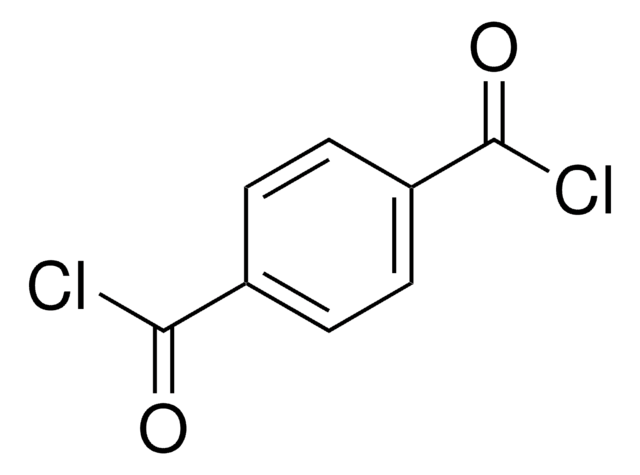
FC6H4CN
CAS Number:
Molecular Weight:
121.11
Beilstein:
2041517
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
99%
양식
solid
bp
188 °C/750 mmHg (lit.)
mp
32-34 °C (lit.)
작용기
fluoro
nitrile
SMILES string
Fc1ccc(cc1)C#N
InChI
1S/C7H4FN/c8-7-3-1-6(5-9)2-4-7/h1-4H
InChI key
AEKVBBNGWBBYLL-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
4-Fluorobenzonitrile undergoes metal-mediated coupling to yield eight-membered thorium(IV) tetraazamacrocycle. It undergoes condensation with diphenylamine to yield monomer 4-cyanotriphenylamine.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Flam. Sol. 1
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point (°F)
149.0 °F - closed cup
Flash Point (°C)
65 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
이미 열람한 고객
Eric J Schelter et al.
Chemical communications (Cambridge, England), (10)(10), 1029-1031 (2007-02-28)
An eight-membered thorium(IV) tetraazamacrocycle is produced by the sequential, metal-mediated coupling of four equivalents of 4-fluorobenzonitrile; its formation is consistent with the involvement of an imido intermediate, generated from a thorium ketimide complex.
Poly (triphenylamine) s derived from oxidative coupling reaction: Substituent effects on the polymerization, electrochemical, and electro-optical properties.
Lin H-Y and Liou G-S.
Journal of Polymer Science Part A: Polymer Chemistry, 47(1), 285-294 (2009)
Soumya Mukherjee et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 24(45), 11771-11778 (2018-05-29)
Fluorous organic building blocks were utilized to develop two self-assembled, hydrophobic, fluorinated porous organic polymers (FPOPs), namely, FPOP-100 and FPOP-101. Comprehensive mechanical analyses of these functionalised triazine network polymers marked the introduction of mechanical stiffness among all porous organic network
Oriol Planas et al.
Science (New York, N.Y.), 367(6475), 313-317 (2020-01-18)
Bismuth catalysis has traditionally relied on the Lewis acidic properties of the element in a fixed oxidation state. In this paper, we report a series of bismuth complexes that can undergo oxidative addition, reductive elimination, and transmetallation in a manner
Tryfon Zarganes-Tzitzikas et al.
Molecules (Basel, Switzerland), 24(7) (2019-04-17)
Imaging techniques, such as positron emission tomography (PET), represent great progress in the clinical development of drugs and diagnostics. However, the efficient and timely synthesis of appropriately labeled compounds is a largely unsolved problem. Numerous small drug-like molecules with high
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.