추천 제품
분석
97%
bp
285 °C (lit.)
mp
52-55 °C (lit.)
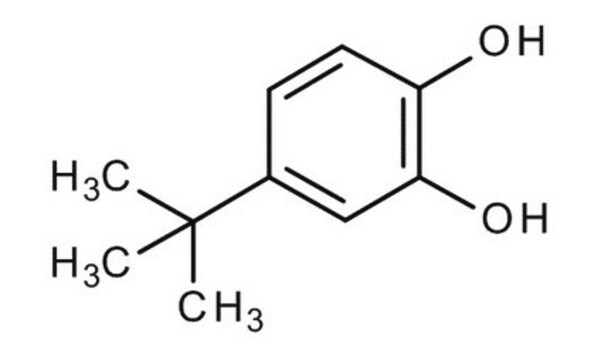
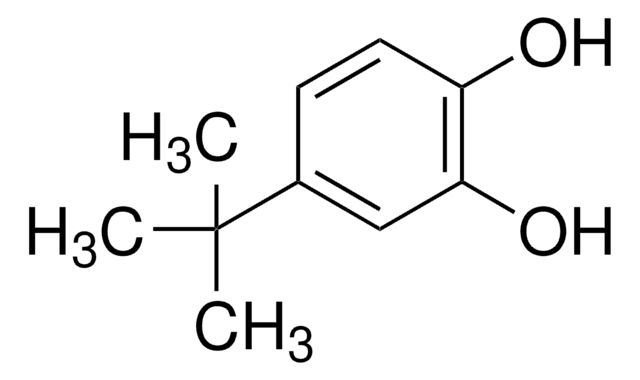
SMILES string
CC(C)(C)c1ccc(O)c(O)c1
InChI
1S/C10H14O2/c1-10(2,3)7-4-5-8(11)9(12)6-7/h4-6,11-12H,1-3H3
InChI key
XESZUVZBAMCAEJ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
4-tert-Butylcatechol undergoes electrochemical trimerization via anodic oxidation and mechanism has been studied by cyclic voltammetry and controlled-potential coulometry. It inhibits the activity of tyrosinase at concentrations higher than 1×10−3M. It undergoes oxidation with laccase to yield quinones which on reaction with dienes and oxidation afford naphthoquinones.
애플리케이션
4-tert-Butylcatechol was used in the synthesis of tungsten oxide nanoparticles by nonaqueous sol-gel process.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Y Usami et al.
Journal of toxicology and environmental health, 6(3), 559-567 (1980-05-01)
4-tert-Butylcatechol (TBC) is an antioxidant widely used in industry and a potent depigmenting agent to the skin of the workers. In this study, tyrosinase was extracted from tissue-cultured human melanoma cells and purified by polyacrylamide gel electrophoresis. T1 and T2
Ligand and solvent effects in the nonaqueous synthesis of highly ordered anisotropic tungsten oxide nanostructures.
Polleux J, et al.
Journal of Materials Chemistry, 16(40), 3969-3975 (2006)
Tetrahedron Letters, 48, 2983-2983 (2007)
Mechanistic study of electrochemical oxidation of 4-tert-butylcatechol: A facile electrochemical method for the synthesis of new trimer of 4-tert-butylcatechol.
Nematollahi D, et al.
Electrochimica Acta, 49(15), 2495-2502 (2004)
J N Rodriguez-López et al.
Analytical biochemistry, 202(2), 356-360 (1992-05-01)
A procedure for calibrating a Clark-type oxygen electrode is described. This method is based on the oxidation of 4-tert-butylcatechol (TBC) by O2 catalyzed by tyrosinase, to yield 4-tert-butyl-o-benzoquinone (TBCQ). This reaction consumes known amounts of oxygen in accordance with the
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.