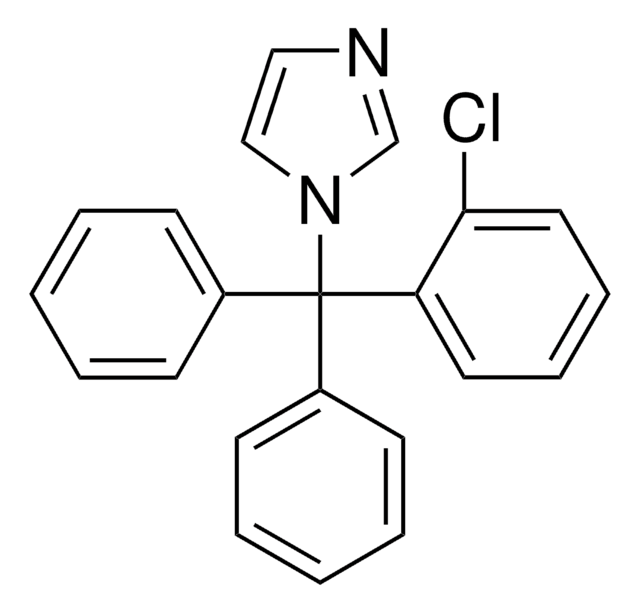
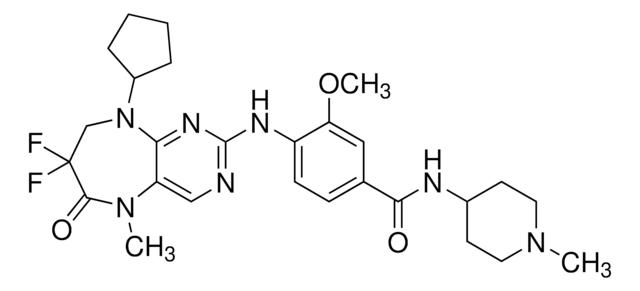
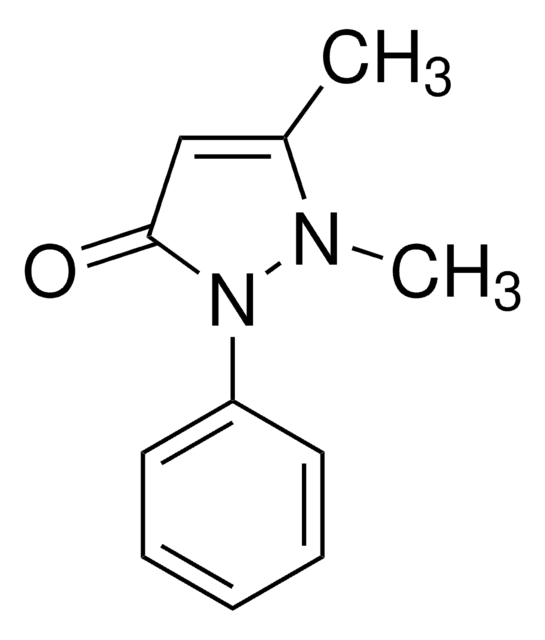
추천 제품
분석
99%
mp
184-186 °C (lit.)
작용기
ketone
SMILES string
CN1N(c2ccccc2)C(=O)C(O)=C1C
InChI
1S/C11H12N2O2/c1-8-10(14)11(15)13(12(8)2)9-6-4-3-5-7-9/h3-7,14H,1-2H3
InChI key
SKVPTPMWXJSBTF-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
4-Hydroxyantipyrine is formed during oxidative deamination of aminopyrine. It is a metabolite of antipyrine.
애플리케이션
4-Hydroxyantipyrine was used to study the relationships between the metabolism of antipyrine, hexobarbitone and theophylline in man. It was used in a study on flow injection analysis system for the characterisation of pharmaceutical compounds via combination of diode array UV, 1H NMR, FT-IR spectroscopy and time-of-flight mass spectrometry.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
M Monshouwer et al.
Xenobiotica; the fate of foreign compounds in biological systems, 25(5), 491-499 (1995-05-01)
1. In order to investigate the effect of a bacterial acute phase response model on drug disposition in vivo, plasma clearances of antipyrine, caffeine, paracetamol and indocyanine green were investigated in the healthy and Actinobacillus pleuropneumoniae-infected pig. 2. Indocyanine green
G Engel et al.
Clinical pharmacology and therapeutics, 59(6), 613-623 (1996-06-01)
Antipyrine has been widely used as a probe drug for human oxidative drug metabolism. To evaluate the role of antipyrine as a model drug, we have identified the cytochrome P450 enzymes involved in 4-hydroxyantipyrine, 3-hydroxymethylantipyrine, and norantipyrine formation. We used
Reaction of drugs with nitrous acid as a source of carcinogenic nitrosamines.
W Lijinsky
Cancer research, 34(1), 255-258 (1974-01-01)
S B Seredenin et al.
Biulleten' eksperimental'noi biologii i meditsiny, 110(11), 491-493 (1990-11-01)
Antipyrine oxidation was studied in C57BL/6 and BALB/c inbred mice. It was found that C57BL/6 are weak oxidant but BALB/c are strong oxidants of antipyrine. Animals F1 hybrids inherited the high capacity of antipyrine oxidation.
R P Shrewsbury et al.
Research communications in chemical pathology and pharmacology, 64(3), 455-462 (1989-06-01)
Antipyrine metabolism was determined after hemodilution with 40 ml/kg of Fluosol in conscious, unrestrained female and male rats. Rats received an intravenous antipyrine dose (20 mg/kg) 24, 48, or 72 hours after hemodilution and the pharmacokinetic parameters were compared to
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.