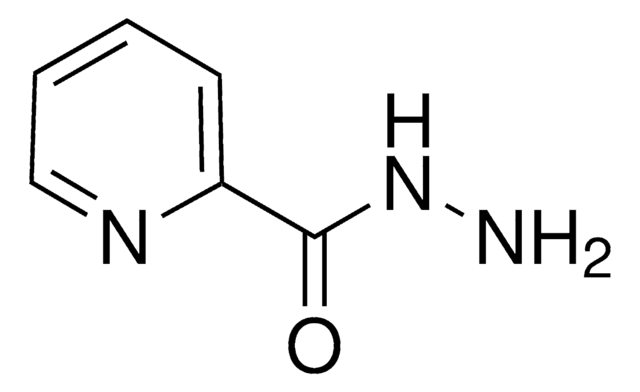
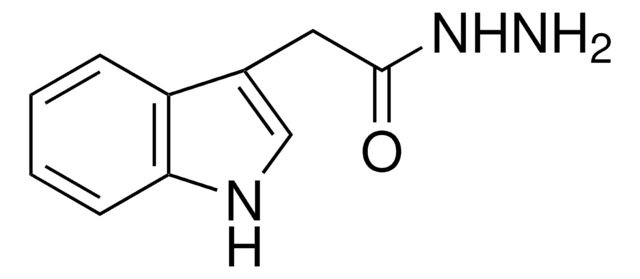
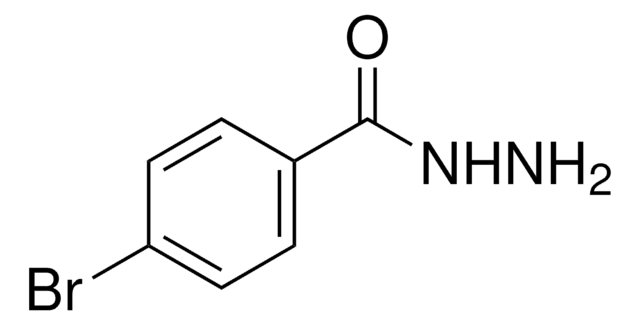
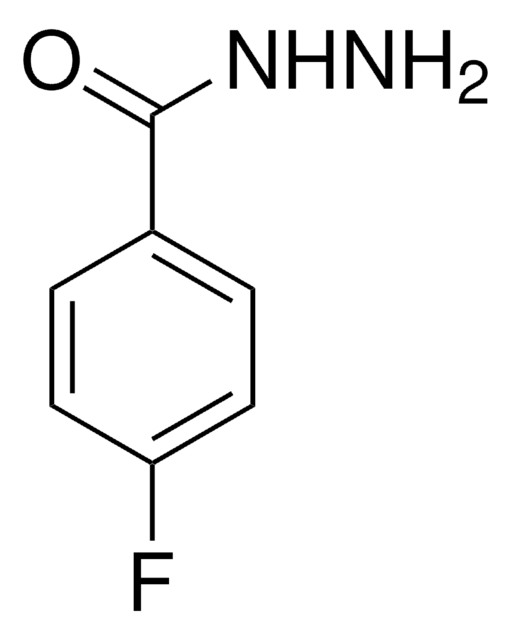
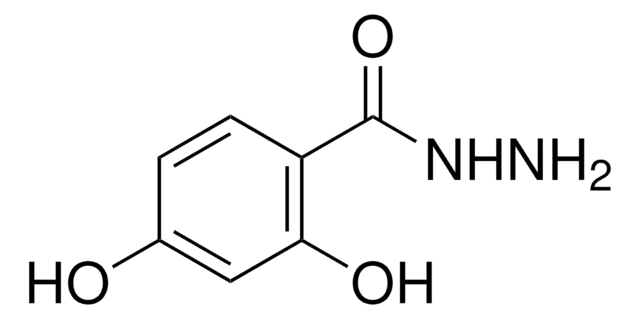
추천 제품
Quality Level
분석
97%
mp
159-161 °C (lit.)
solubility
H2O: soluble 50 mg/mL
작용기
amine
hydrazine
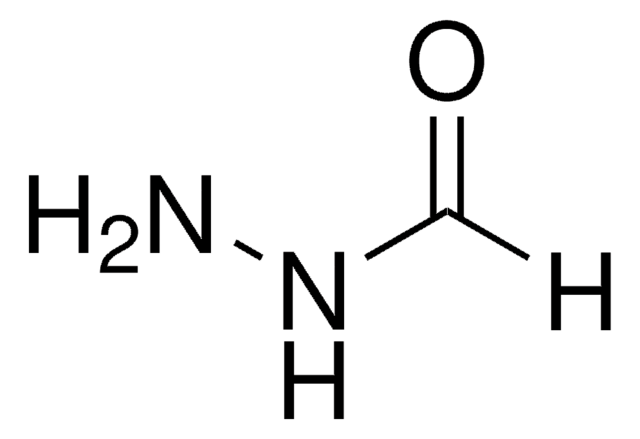
SMILES string
NNC(=O)c1cccnc1
InChI
1S/C6H7N3O/c7-9-6(10)5-2-1-3-8-4-5/h1-4H,7H2,(H,9,10)
InChI key
KFUSANSHCADHNJ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Nicotinic hydrazide is a heterocyclic compound that can be used to synthesize Schiff bases.
애플리케이션
Nicotinic hydrazide was used in hydrazone library formation. It was used to study the oxidation of isonicotinic acid hydrazide (isoniazid) by horseradish peroxidase.
생화학적/생리학적 작용
Nicotinic hydrazide is an inhibitor of peroxidase enzyme. It forms solid metal complexes having strong biological activity.
제조 메모
Nicotinic hydrazide dissolves in water at a concentration of 50 mg/ml to form a clear, colourless solution.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
V Goral et al.
Proceedings of the National Academy of Sciences of the United States of America, 98(4), 1347-1352 (2001-02-15)
Dynamic combinatorial libraries are mixtures of compounds that exist in a dynamic equilibrium and can be driven to compositional self adaptation via selective binding of a specific assembly of certain components to a molecular target. We present here an extension
Thermo-chemical behavior of solid nicotinic hydrazide metal complexes in correlation with their stoichiometry.
Sekkina MM and El-Azm MG.
Thermochimica Acta, 77(1), 211-218 (1984)
H A Shoeb et al.
Antimicrobial agents and chemotherapy, 27(3), 399-403 (1985-03-01)
Oxidation of isonicotinic acid hydrazide (isoniazid) by horseradish peroxidase at the expense of H2O2 yielded reactive species which were able to reduce nitroblue tetrazolium and bleach p-nitrosodimethylaniline. Nicotinic acid hydrazide oxidation did not cause these effects. At slightly alkaline pH
Alireza Moradi et al.
Archiv der Pharmazie, 343(9), 509-518 (2010-09-02)
A series of 2-phenoxynicotinic acid hydrazides were synthesized and evaluated for their analgesic and anti-inflammatory activities. Several compounds having an unsubstituted phenyl/4-pyridyl or C-4 methoxy substituent on the terminal phenyl ring showed moderate to high analgesic or anti-inflammatory activity in
[Tautomerism of 2-hydrazino-4-phenylthiazole<-->4-phenylthiazol-2-one hydrazone. Derivatives of acids. II. (4-phenyl-3-R-thiazol-2-ylidene) and beta-methyl-beta-(4-phenylthiazol-2-yl) hydrazides of picolinic, nicotinic, and isonicotinic acid].
L Bielak et al.
Annales Universitatis Mariae Curie-Sklodowska. Sectio D: Medicina, 44, 41-51 (1989-01-01)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.