すべての画像(1)
About This Item
実験式(ヒル表記法):
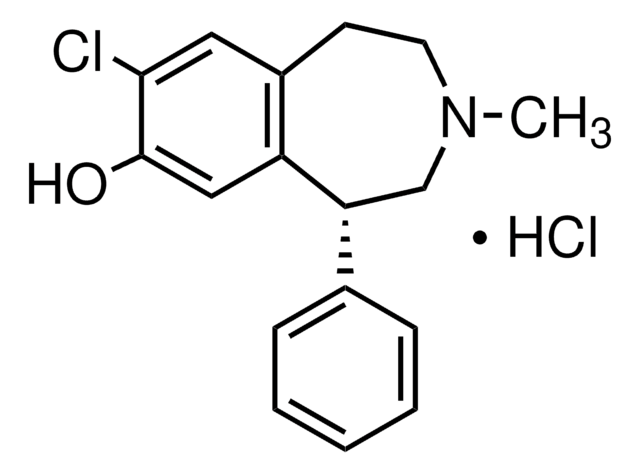
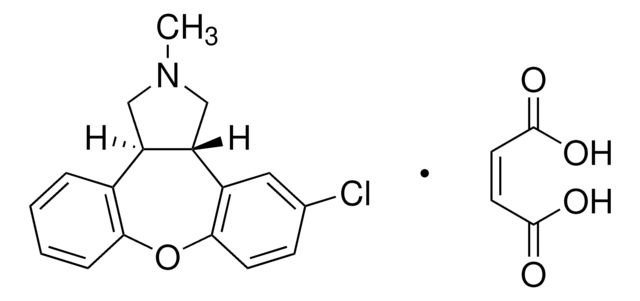
C16H17NO2 · HCl
CAS番号:
分子量:
291.77
MDL番号:
UNSPSCコード:
12352200
PubChem Substance ID:
NACRES:
NA.77
おすすめの製品
品質水準
アッセイ
≥98% (HPLC)
フォーム
solid
光学活性
[α]22/D +16.1°, c = 1.2 in methanol(lit.)
保管条件
desiccated
色
off-white to light tan
溶解性
0.1 M HCl: 1.2 mg/mL
ethanol: 3.4 mg/mL
H2O: 5 mg/mL
aqueous base: unstable
SMILES記法
Cl.Oc1cc2CCNC[C@H](c3ccccc3)c2cc1O
InChI
1S/C16H17NO2.ClH/c18-15-8-12-6-7-17-10-14(13(12)9-16(15)19)11-4-2-1-3-5-11;/h1-5,8-9,14,17-19H,6-7,10H2;1H/t14-;/m1./s1
InChI Key
YEWHJCLOUYPAOH-PFEQFJNWSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
アプリケーション
(R)-(+)-SKF-38393 hydrochloride has been used as a D1 dopamine receptor agonist to study its effect on the sleep-wake pattern of macaques rendered parkinsonian with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).
生物化学的/生理学的作用
(R)-(+)-SKF-38393 hydrochloride is a D1 dopamine receptor agonist and an active enantiomer of (±)-SKF-38393. It enhances pertussis toxin-insensitive and protein kinase A-mediated glutamate release in the hippocampal neurons. SKF-38393, a benzazepine derivative, exhibits anorectic effects.
特徴および利点
This compound is featured on the Dopamine Receptors page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
注意
光に不安定です。
再構成
溶液は、4°Cで数日間は保存することができます。
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
dust mask type N95 (US), Eyeshields, Gloves
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
S101-25MG:
S101-IP:
S101-VAR:
S101-100MG:
S101-BULK:
S101-5MG:
この製品を見ている人はこちらもチェック
Carole Hyacinthe et al.
Neurobiology of disease, 63, 20-24 (2013-11-12)
Both excessive daytime sleepiness (EDS) and rapid eye movement (REM) sleep deregulation are part of Parkinson's disease (PD) non-motor symptoms and may complicate dopamine replacement therapy. We report here that dopamine agonists act differentially on sleep architecture in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
Steven J Cooper et al.
European journal of pharmacology, 532(3), 253-257 (2006-02-16)
Free-feeding rats meet much of their daily energy requirements by consuming food in meals during the nocturnal phase of the night/day cycle. Meal pattern analysis methodology has been developed to record the patterns of meal taken over a 24-h period
C Kaiser et al.
Journal of medicinal chemistry, 25(6), 697-703 (1982-06-01)
Resolution of the unique dopamine receptor agonist 2,3,4,5-tetrahydro-7,8-dihydroxy-1-phenyl-1H-3-benzazepine (1) was achieved by a stereospecific multistep conversion of the readily separated enantiomers of its O,O,N-trimethylated precursor 2. The absolute stereochemistry of the antipodes of 2-MeI was determined by single-crystal X-ray diffractometric
J L Katz et al.
Psychopharmacology, 107(2-3), 217-220 (1992-01-01)
The present study was designed to assess the behavioral similarity of the effects of prototype dopamine receptor-subtype selective agonists and cocaine. Squirrel monkeys (N = 4) were trained with food reinforcement to press one of two levers after administration of
E Ongini et al.
Life sciences, 37(24), 2327-2333 (1985-12-16)
The dopamine D-1 receptor agonist SKF 38393 dose-dependently (2.5-10 mg/kg) induced desynchronization of the electroencephalographic (EEG) activity and behavioral arousal in both rabbits and rats. Unlike apomorphine, SKF 38393 elicited no signs of stereotyped behavior in rabbits and minimal effects
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)