おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
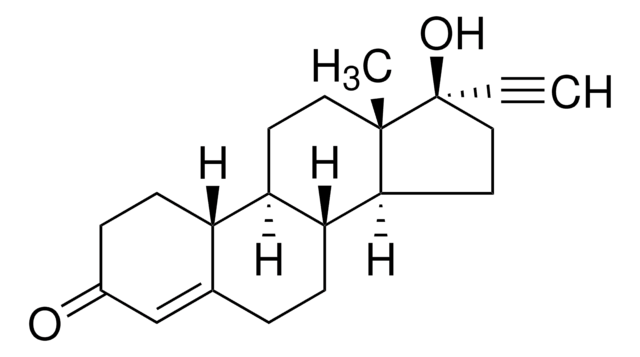
lynestrenol
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
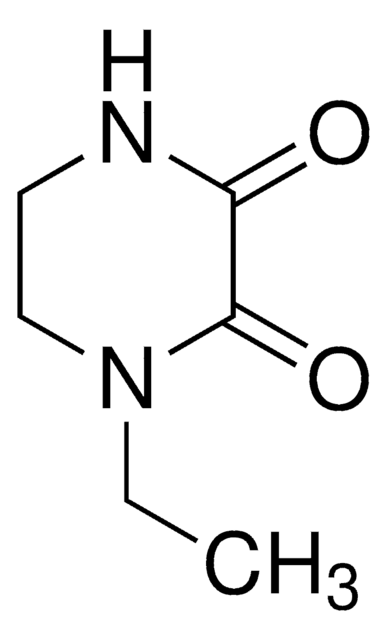
SMILES記法
O[C@@]1([C@@]2([C@H]([C@H]3[C@@H]([C@H]4CCCC=C4CC3)CC2)CC1)C)C#C
InChI
1S/C20H28O/c1-3-20(21)13-11-18-17-9-8-14-6-4-5-7-15(14)16(17)10-12-19(18,20)2/h1,6,15-18,21H,4-5,7-13H2,2H3/t15-,16+,17+,18-,19-,20-/m0/s1
InChI Key
YNVGQYHLRCDXFQ-XGXHKTLJSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
この製品は薬局方標準品です。発行元の薬局方により製造・供給されています。MSDSを含む製品情報などの詳しい情報は、発行元の薬局方のウェブサイトよりご確認ください。
アプリケーション
Lynestrenol for peak identification EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包装
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
その他情報
Sales restrictions may apply.
最新バージョンのいずれかを選択してください:
M Iqbal Choudhary et al.
Natural product research, 24(1), 1-6 (2009-12-17)
Transformation of lynestrenol (19-nor-17alpha-pregn-4-en-20-yn-17beta-ol) (1) was carried out by incubation with Cunninghamella elegans to obtain 19-nor-17alpha-pregn-4-en-20-yn-3-one-10beta,17beta-diol (2), 19-nor-17alpha-pregn-4-en-20-yn-3-one-6beta,17beta-diol (3), and 19-nor-17alpha-pregn-4-en-20-yn-3beta,6beta,17beta-triol (4). Metabolite 4 was identified as a new compound. These metabolites were structurally characterised on the basis of spectroscopic
Dominik Rachoń et al.
Menopause (New York, N.Y.), 13(5), 840-845 (2006-08-09)
Oral estrogen increases the levels of C-reactive protein (CRP), which is an independent risk factor for coronary heart disease in healthy individuals. The aim of our study was to investigate the effects of intranasal 17beta-estradiol (E2) on serum CRP and
Venous thromboembolism after high-dose chemotherapy in a patient with Hodgkin's lymphoma receiving the new oral contraceptive ethinylestradiol and drospirenone ("Yasmine").
A Tartarone et al.
Bone marrow transplantation, 35(1), 103-103 (2004-11-09)
Ofer Amir et al.
Journal of speech, language, and hearing research : JSLHR, 49(5), 1114-1126 (2006-11-02)
Two studies are presented here. Study 1 was aimed at evaluating whether the voice characteristics of women who use birth control pills that contain different progestins differ from the voice characteristics of a control group. Study 2 presents a meta-analysis
Sidsel Graff-Iversen et al.
Contraception, 66(1), 7-13 (2002-08-10)
This population-based cross-sectional study assessed lipid and lipoprotein parameters in women using progestogen-only contraceptives or medications and in those using no hormones. Unselected women about age 40 to 42 years were invited, and the participation rate was 65.6%. A total
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)