おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
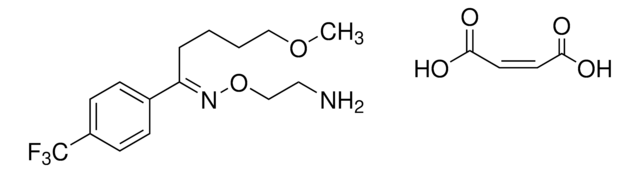
fluvoxamine
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
SMILES記法
COCCCC/C(C1=CC=C(C(F)(F)F)C=C1)=N\OCCN.OC(/C=C\C(O)=O)=O
InChI
1S/C15H21F3N2O2.C4H4O4/c1-21-10-3-2-4-14(20-22-11-9-19)12-5-7-13(8-6-12)15(16,17)18;5-3(6)1-2-4(7)8/h5-8H,2-4,9-11,19H2,1H3;1-2H,(H,5,6)(H,7,8)/b20-14+;2-1-
InChI Key
LFMYNZPAVPMEGP-PIDGMYBPSA-N
遺伝子情報
human ... SLC6A4(6532)
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
この製品は薬局方標準品です。発行元の薬局方により製造・供給されています。MSDSを含む製品情報などの詳しい情報は、発行元の薬局方のウェブサイトよりご確認ください。
アプリケーション
Fluvoxamine maleate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包装
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
その他情報
Sales restrictions may apply.
関連製品
製品番号
詳細
価格
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
Criteria for use of fluvoxamine maleate in adult inpatients and outpatients.
American journal of hospital pharmacy, 51(18), 2282-2285 (1994-09-15)
Adele Stewart et al.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 28(4), 1735-1744 (2014-01-15)
Targeting serotonin (5-HT) bioavailability with selective 5-HT reuptake inhibitors (SSRIs) remains the most widely used treatment for mood disorders. However, their limited efficacy, delayed onset of action, and side effects restrict their clinical utility. Endogenous regulator of G-protein signaling (RGS)
Tomohisa Mori et al.
The Journal of pharmacology and experimental therapeutics, 350(2), 403-411 (2014-06-12)
Previous studies have demonstrated that methylphenidate, MDMA (3,4-methylenedioxymethamphetamine), and other psychostimulants exert stimulant-like subjective effects in humans. Furthermore, MDMA and methylphenidate substitute for the discriminative stimulus effects of psychostimulants, such as amphetamine and cocaine, in animals, which suggests that MDMA
Loqman A Mohamed et al.
Journal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques, 17(3), 427-438 (2014-09-17)
PURPOSE. The knowledge of hepatic disposition kinetics of tacrine, a first cholinesterase inhibitor was approved by FDA for the treatment of Alzheimer's disease (AD), would help to understand its hepatotoxicity, its therapeutic effect, and improve the management of patients with
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)