おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
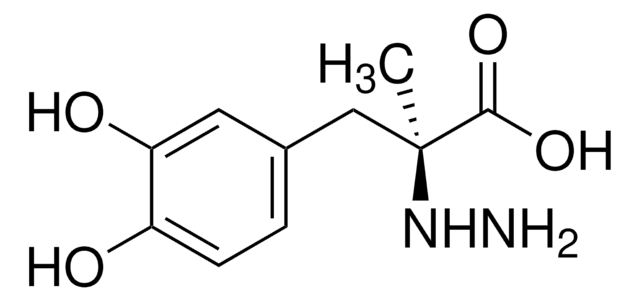
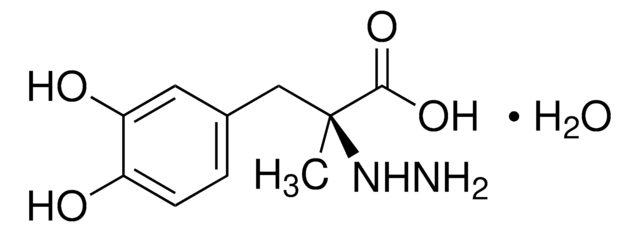
carbidopa
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
SMILES記法
O.C[C@@](Cc1ccc(O)c(O)c1)(NN)C(O)=O
InChI
1S/C10H14N2O4.H2O/c1-10(12-11,9(15)16)5-6-2-3-7(13)8(14)4-6;/h2-4,12-14H,5,11H2,1H3,(H,15,16);1H2/t10-;/m0./s1
InChI Key
QTAOMKOIBXZKND-PPHPATTJSA-N
遺伝子情報
human ... DDC(1644)
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
Carbidopa is a dopaminergic drug, which is known to inhibit the conversion of dopa to dopamine. It is used in combination with levodopa for the effective treatment of restless legs syndrome and periodic leg movements in sleep.
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
アプリケーション
Carbidopa EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包装
この製品は発行元の薬局方による供給としてお届けします。現在の単位量については、EDQM reference substance catalogueをご覧ください。
その他情報
Sales restrictions may apply.
関連製品
製品番号
詳細
価格
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral - Eye Irrit. 2
保管分類コード
11 - Combustible Solids
WGK
WGK 3
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
C0460000-1EA:
C0460000:
C0460000-50MG:
最新バージョンのいずれかを選択してください:
この製品を見ている人はこちらもチェック
Àlex Pericas et al.
Organic letters, 15(7), 1448-1451 (2013-03-13)
A stereoselective synthesis of L-carbidopa in seven steps and 50% overall yield from commercial compounds is described. The key step involves a highly enantioselective α-amination reaction of an acyclic β-ketoester with di-tert-butyl azodicarboxylate induced by europium and (R,R)-diphenyl-pybox.
Augmentation of the restless legs syndrome with carbidopa/levodopa
Allen.PR and Earley.JC
Sleep, 19(3), 205-213 (1996)
Levodopa-carbidopa intestinal gel in advanced Parkinson's disease open-label study: interim results.
Hubert H Fernandez et al.
Parkinsonism & related disorders, 19(3), 339-345 (2013-01-05)
Levodopa-carbidopa intestinal gel (LCIG) delivered continuously via percutaneous endoscopic gastrojejunostomy (PEG-J) tube has been reported, mainly in small open-label studies, to significantly alleviate motor complications in Parkinson's disease (PD). A prospective open-label, 54-week, international study of LCIG is ongoing in
Ina Schabram et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 34(44), 14769-14776 (2014-10-31)
Methylphenidate (MPH) inhibits the reuptake of dopamine and noradrenaline. PET studies with MPH challenge show increased competition at postsynaptic D2/3-receptors, thus indirectly revealing presynaptic dopamine release. We used [(18)F]fluorodopamine ([(18)F]FDOPA)-PET in conjunction with the inlet-outlet model (IOM) of Kumakura et
Kathleen Fagerlund et al.
The American journal of nursing, 113(1), 26-35 (2012-12-19)
Carbidopa-levodopa (Sinemet), the gold-standard treatment for Parkinson's disease, has a short half-life of one to two hours. When patients with Parkinson's disease are placed on NPO (nil per os, or nothing by mouth) status for surgery, they may miss several
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)