おすすめの製品
グレード
pharmaceutical primary standard
認証
BP
APIファミリー
famotidine
フォーム
solid
シェルフライフ
limited shelf life, expiry date on the label
包装
pkg of 100 mg
メーカー/製品名
BP
保管条件
OK to freeze
色
white
mp
162-165 °C
溶解性
water: insoluble
アプリケーション
pharmaceutical
pharmaceutical small molecule
フォーマット
neat
輸送温度
ambient
保管温度
2-8°C
SMILES記法
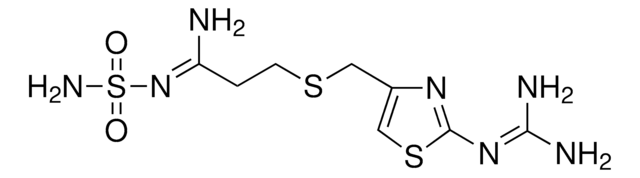
N\C(N)=N\c1nc(CSCCC(=N)NS(N)(=O)=O)cs1
InChI
1S/C8H15N7O2S3/c9-6(15-20(12,16)17)1-2-18-3-5-4-19-8(13-5)14-7(10)11/h4H,1-3H2,(H2,9,15)(H2,12,16,17)(H4,10,11,13,14)
InChI Key
XUFQPHANEAPEMJ-UHFFFAOYSA-N
遺伝子情報
human ... HRH2(3274)
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
Famotidine is a hydrophilic, cationic, histamine H2 receptor antagonist drug that effectively inhibits gastric acid secretion in humans.
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
アプリケーション
Famotidine BP Reference standard, intended for use in laboratory tests only as specifically prescribed in the British Pharmacopoeia.
Also used in monographs such as:Famotidine Tablets
Also used in monographs such as:
生物化学的/生理学的作用
H2ヒスタミンレセプタ-のアンタゴニストであり、抗潰瘍薬です。
包装
Unit quantity: 100 mg. Subject to change. The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity please visit British Pharmacopoeia
その他情報
Sales restrictions may apply.
関連製品
製品番号
詳細
価格
保管分類コード
11 - Combustible Solids
WGK
WGK 2
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
BP653:
最新バージョンのいずれかを選択してください:
Famotidine
British Pharmacopeia
British Pharmacopoeia, 29(1-2), 247-254 (2020)
Stability indicating method for famotidine in pharmaceuticals using porous graphitic carbon column
Helali N and Monser L
Journal of Separation Science, 31(2) (2008)
Capillary zone electrophoresis method for the determination of famotidine and related impurities in pharmaceuticals
Helali N, et al.
Talanta, 74(4) (2008)
Potentiometric determination of famotidine in pharmaceutical formulations
Ayad MM, et al.
Journal of Pharmaceutical and Biomedical Analysis, 29(1-2) (2002)
Determination of cimetidine, famotidine, and ranitidine hydrochloride in the presence of their sulfoxide derivatives in pure and dosage forms by high-performance thin-layer chromatography and scanning densitometry
Kelani KM, et al.
Journal of AOAC (Association of Official Analytical Chemists) International, 85(5), 1015-1020 (2002)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)