おすすめの製品
詳細
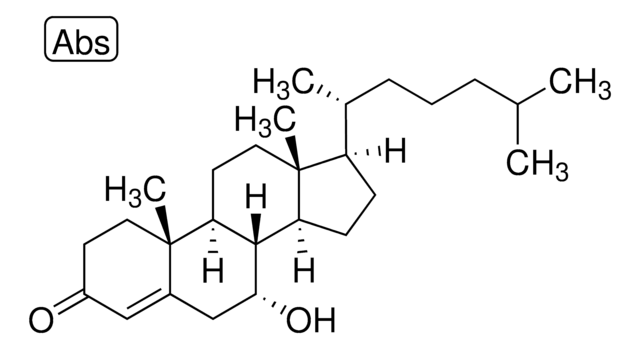
7α-hydroxy-4-cholesten-3-one
アッセイ
>99% (TLC)
フォーム
powder
包装
pkg of 1 × 1 mg (700113P-1mg)
pkg of 1 × 10 mg (700113P-10mg)
メーカー/製品名
Avanti Research™ - A Croda Brand
輸送温度
dry ice
保管温度
−20°C
InChI
1S/C27H44O2/c1-17(2)7-6-8-18(3)21-9-10-22-25-23(12-14-27(21,22)5)26(4)13-11-20(28)15-19(26)16-24(25)29/h15,17-18,21-25,29H,6-14,16H2,1-5H3/t18-,21-,22+,23+,24-,25+,26+,27-/m1/s1
InChI Key
IOIZWEJGGCZDOL-RQDYSCIWSA-N
関連するカテゴリー
詳細
7α-Hydroxy-4-cholesten-3-one is an intermediate in the biochemical synthesis of bile acids from cholesterol. Its precursor, 7α-hydroxycholesterol, is produced from cholesterol by hepatic cholesterol 7α-hydroxylase (CYP7A1).[1]It is metabolized by the enzyme 7α-hydroxycholest-4-en-3-one 12α-hydroxylase to 7α,12α-dihydroxycholest-4-en-3-one and then to cholic acid, the major primary bile acid in humans. Alternatively, it can be converted into 5β-cholestane-3α,7α-diol and then to chenodeoxycholic acid, the other major primary bile acid in humans.Serum 7α-hydroxy-4-cholesten-3-one concentrations reflect the activity of the bile acid synthetic pathway. Serum 7α-hydroxy-4-cholesten-3-one values vary during the day as bile acid synthetic rates have a diurnal rhythm.[2]Elevated values are found in patients with bile acid malabsorption and may be useful in the diagnosis of this condition as high values are associated with low SeHCAT retention.[3] The increase in serum 7α-hydroxy-4-cholesten-3-one concentrations reflects the loss of bile acids secondary to bile acid malabsorption or the increased synthesis found in primary bile acid diarrhea associated with impaired negative feedback of CYP7A1 by FGF19.[4]
アプリケーション
- Role as a mediator in cholesterol metabolism: 7α-hydroxycholestenone has been implicated in the discussion on oxysterols as cholesterol metabolites that act as significant mediators within biological systems, highlighting its importance in cholesterol metabolism and potential pharmaceutical implications (Mutemberezi et al., 2016).
包装
5 mL Amber Glass Screw Cap Vial (700113P-10mg)
5 mL Amber Glass Screw Cap Vial (700113P-1mg)
法的情報
Avanti Research is a trademark of Avanti Polar Lipids, LLC
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
700113P-VAR:
700113P-10MG:
700113P-1MG:
700113P-BULK:
最新バージョンのいずれかを選択してください:
Serum 7 alpha-hydroxy-4-cholesten-3-one and selenohomocholyltaurine (SeHCAT) whole body retention in the assessment of bile acid induced diarrhoea.
Brydon WG, et al.
European Journal of Gastroenterology & Hepatology, 8(2), 117-123 (1996)
The enzymes, regulation, and genetics of bile acid synthesis. Annual review of biochemistry
Russell DW
Annual Review of Biochemistry, 72, 137-174 (2003)
Chronic diarrhea due to excessive bile acid synthesis and not defective ileal transport: a new syndrome of defective fibroblast growth factor 19 release.
Hofmann AF, et al.
Clinical Gastroenterology and Hepatology : the Official Clinical Practice Journal of the American Gastroenterological Association, 7 (11), 1151-1154 (2009)
Bile acid synthesis in humans has a rapid diurnal variation that is asynchronous with cholesterol synthesis.
Galman C, et al.
Gastroenterology, 129(5), 1445-1453 (2005)
Cecilia Gälman et al.
Gastroenterology, 129(5), 1445-1453 (2005-11-16)
The conversion of cholesterol to bile acids by the liver is an important regulator of body cholesterol homeostasis. In rodents, both cholesterol and bile acid synthesis have marked diurnal rhythms that peak synchronously at midnight. The aim of this study
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)