533610
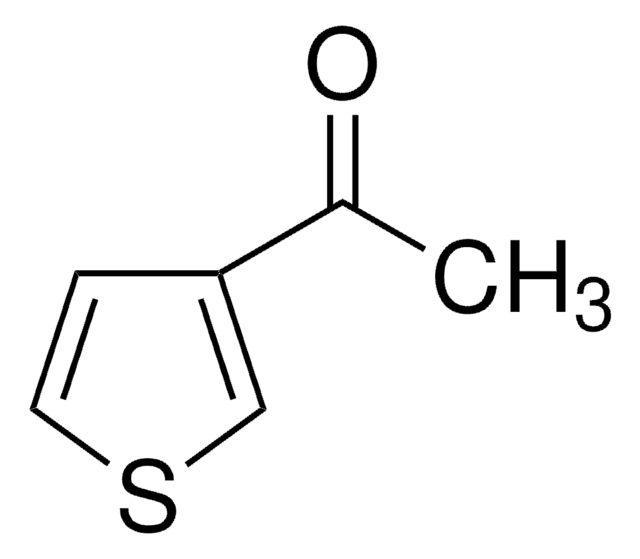
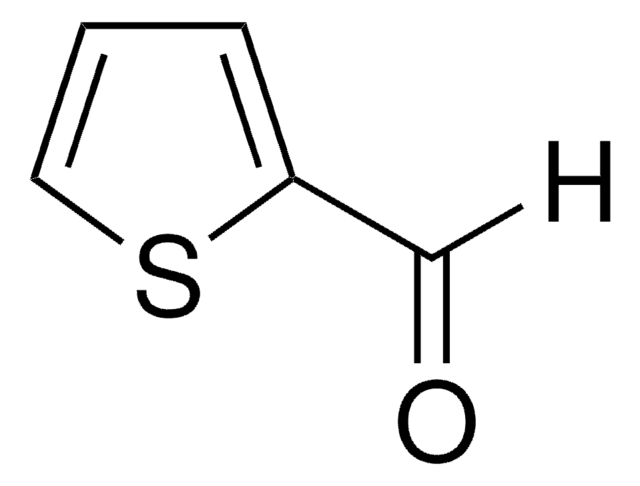
2-アセチル-5-メチルチオフェン
98%
別名:
1-(5-Methyl-2-thienyl)ethanone, 1-(5-Methylthiophen-2-yl)ethan-1-one, 1-(5-Methylthiophen-2-yl)ethanone, 2-Methyl-5-acetylthiophene, 5-Methyl-2-thienyl methyl ketone, Methyl 5-methyl-2-thienyl ketone
ログイン組織・契約価格を表示する
すべての画像(1)
About This Item
実験式(ヒル表記法):
C7H8OS
CAS番号:
分子量:
140.20
EC Number:
MDL番号:
UNSPSCコード:
12352100
PubChem Substance ID:
NACRES:
NA.22
おすすめの製品
アッセイ
98%
屈折率
n20/D 1.561 (lit.)
bp
65-67 °C/1 mmHg (lit.)
mp
24-28 °C (lit.)
密度
1.106 g/mL at 25 °C (lit.)
官能基
ketone
SMILES記法
CC(=O)c1ccc(C)s1
InChI
1S/C7H8OS/c1-5-3-4-7(9-5)6(2)8/h3-4H,1-2H3
InChI Key
YOSDTJYMDAEEAZ-UHFFFAOYSA-N
詳細
2-Acetyl-5-methylthiophene is a volatile organic compound formed during the reaction between L-cysteine and dihydroxyacetone in glycerine or triglyceride solvent system. It can be prepared by reacting 2-methylthiophene with acetic anhydride. 2-Acetyl-5-methylthiophene undergoes palladium-catalyzed cross-coupling reaction with aryl bromides to form C-4 arylated product. It reacts with 1,2-bis(5-formyl-2-methylthiophen-3-yl)cyclopentene via Aldol condensation to form a chalcone with photochromic property. The standard molar enthalpies of combustion, formation and vaporization of 2-acetyl-5-methylthiophene are 4341.9 ± 1.8kJ/mol, 158.0 ± 2.1kJ/mol and 62.0 ± 2.6kJ/mol, respectively.
アプリケーション
2-Acetyl-5-methylthiophene may be used in the preparation of:
- 2-ethyl-5-methylthiophene
- (5-methylthiophen-2-yl)glyoxal
- (2E)-1-(5-methylthiophen-2-yl)-3-(pyridin-3-yl)prop-2-en-1-one
- ethyl 3-(5-methylthiophen-2-yl)-3-oxopropanoate
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Eyeshields, Gloves, type N95 (US)
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
533610-BULK:
533610-VAR:
533610-25G:
Palladium-catalysed direct 3-or 4-arylation of thiophene derivatives using aryl bromides.
Dong JJ, et al.
Tetrahedron, 50(23), 2778-2781 (2009)
Zinc azaphthalocyanines with thiophen-2-yl, 5-methylthiophen-2-yl and pyridin-3-yl peripheral substituents: Additive substituent contributions to singlet oxygen production.
M?rkved EH, et al.
Dyes and Pigments, 82(3), 276-285 (2009)
Novel Dithienylethenes with Extended ??Systems: Synthesis by Aldol Condensation and Photochromic Properties.
Altenhoner K, et al.
European Journal of Organic Chemistry, 2010(31), 6033-6037 (2010)
Experimental thermochemical study of the three methyl substituted 2-acetylthiophene isomers.
da Silva MAVR and Santos AFLOM.
The Journal of Chemical Thermodynamics, 40(8), 1309-1313 (2008)
Efficient guaiazulene and chamazulene syntheses involving [6+4] cycloadditions.
Mukherjee D, et al.
Journal of the American Chemical Society, 1010(1), 251-252 (1979)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)