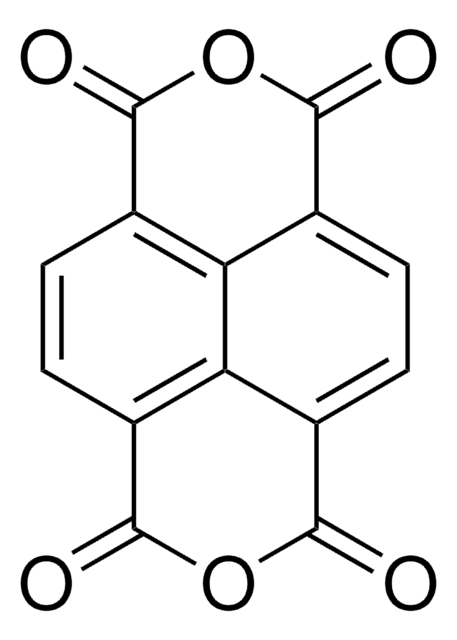
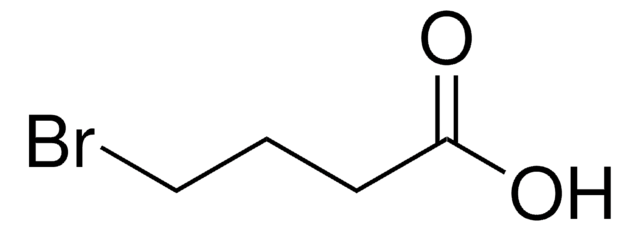
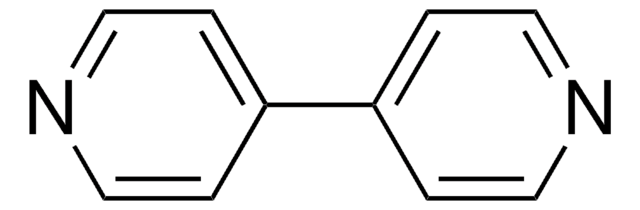
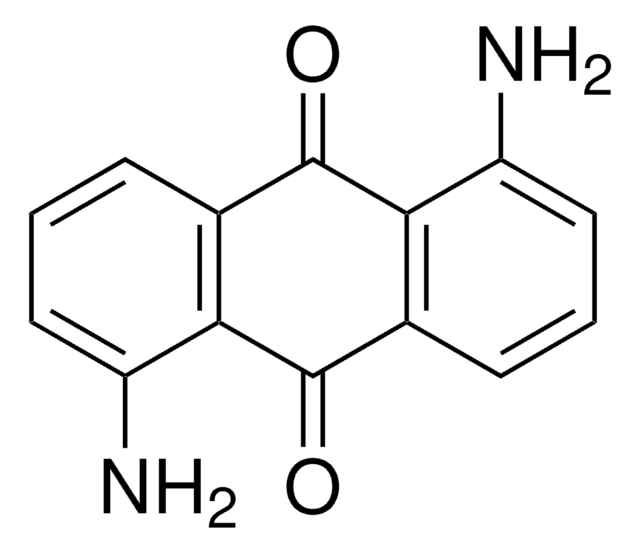
おすすめの製品
アッセイ
>83.0% (HPLC)
品質水準
形状
powder or crystals
mp
>300 °C (lit.)
アプリケーション
diagnostic assay manufacturing
hematology
histology
保管温度
room temp
SMILES記法
[Na+].Nc1c(cc(Br)c2C(=O)c3ccccc3C(=O)c12)S([O-])(=O)=O
InChI
1S/C14H8BrNO5S.Na/c15-8-5-9(22(19,20)21)12(16)11-10(8)13(17)6-3-1-2-4-7(6)14(11)18;/h1-5H,16H2,(H,19,20,21);/q;+1/p-1
InChI Key
TXMRAEGWZZVGIH-UHFFFAOYSA-M
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Eye Irrit. 2
保管分類コード
11 - Combustible Solids
WGK
WGK 1
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
dust mask type N95 (US), Eyeshields, Gloves
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Yuanyuan Qu et al.
FEMS microbiology letters, 246(1), 143-149 (2005-05-05)
Sphingomonas xenophaga QYY with the ability to degrade bromoamine acid (BAA) was previously isolated from sludge samples. The enhancement of BAA removal by strain QYY in sequencing batch reactors (SBRs) was investigated in this study. The results showed that augmented
Yuan-yuan Qu et al.
Applied biochemistry and biotechnology, 159(3), 664-672 (2009-01-14)
A combined biological (augmented membrane bioreactor (MBR)) and photochemical (photocatalysis and ozonation) treatment has been proposed for bromoamine acid (BAA) removal in dyeing wastewater. It was demonstrated that the color and chemical oxygen demand removal in the sequential augmented MBR
Wei Zhang et al.
Huan jing ke xue= Huanjing kexue, 30(10), 2930-2935 (2009-12-09)
Combined ALR-BAC was used to treat bromoamine acid wastewater. The results showed that the ALR system could run steadily for over 1 months at the BAA concentration 650 mg x L(-1) after one-month acclimation, the decoloration rate of BAA was
Yuanyuan Qu et al.
Biodegradation, 17(1), 83-91 (2006-02-03)
One high-effective bromoamine acid (1-amino-4-bromoanthraquinone-2-sulfonic acid, BAA) degrading strain was isolated previously with the ability to use BAA as sole source of carbon and nitrogen. It was identified as Sphingomonas xenophaga QYY by 16S rDNA sequence analysis and physio-biochemical tests.
Younis Baqi et al.
Organic letters, 9(7), 1271-1274 (2007-03-14)
[structure: see text]. The synthesis of anilinoanthraquinones 3a-z was achieved by a new, Cu(0)-catalyzed, microwave-assisted Ullmann coupling reaction of bromaminic acid (1) with aniline derivatives 2a-z in phosphate buffer. Good to excellent isolated yields were obtained within only 2-20 min
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)

![1,3-ビス[4-(ジメチルアミノ)フェニル]-2,4-ジヒドロキシシクロブテンジイリウム 二水酸化物, ビス(分子内塩) Dye content 90 %](/deepweb/assets/sigmaaldrich/product/structures/301/519/500149b3-198c-44cf-b952-7e91f54fc48e/640/500149b3-198c-44cf-b952-7e91f54fc48e.png)