Key Documents
Safety Information
1.37037
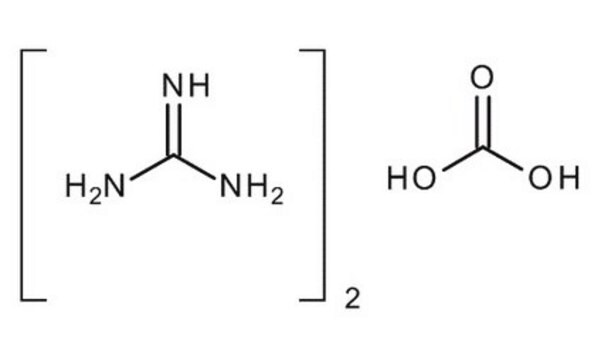
Guanidinium chloride
EMPROVE® EXPERT, NF
Pharma Manufacturing
Synonym(s):
Guanidine hydrochloride, Guanidinum hydrochloride, Carbamimidoylazanium chloride, Aminoformamidine hydrochloride, Aminomethanamidine hydrochloride, Guanidinium chloride
About This Item
Recommended Products
Agency
NF
Quality Level
product line
EMPROVE® EXPERT
potency
655.3-907.1 mg/kg LD50, oral (Rat)
>2000 mg/kg LD50, skin (Rabbit)
feature
RNase and DNase free
pH
4.5-5.5 (20 °C, 100 g/L in H2O)
mp
180-185 °C (lit.)
188 °C
solubility
2150 g/L
density
1.3 g/cm3 (lit.)
bulk density
550‑620 kg/m3
storage temp.
15-25°C
SMILES string
Cl[H].NC(N)=N
InChI
1S/CH5N3.ClH/c2-1(3)4;/h(H5,2,3,4);1H
InChI key
PJJJBBJSCAKJQF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
M-Clarity Program
As part of our EMPROVE® Program, our raw materials are offered with EMPROVE® Dossiers which provide comprehensive, up-to-date documentation to help you navigate regulatory challenges, manage risks, and improve your manufacturing processes.
Our comprehensive portfolio of downstream process chemicals not only provides biopharmaceutical manufacturers with high-quality raw materials for production of classical and novel therapies, but also helps them get to market faster and simplify regulatory challenges. Ranging from non-GMP grades for low-risk application, to IPEC-PQG GMP for higher-risk applications, we have products covering all your manufacturing needs.
Application
Other Notes
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service