347817
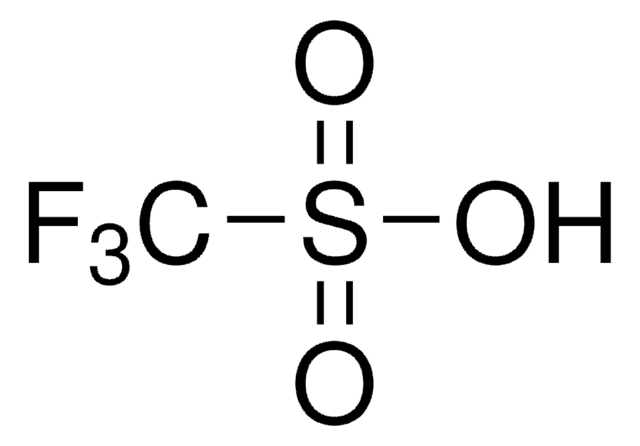
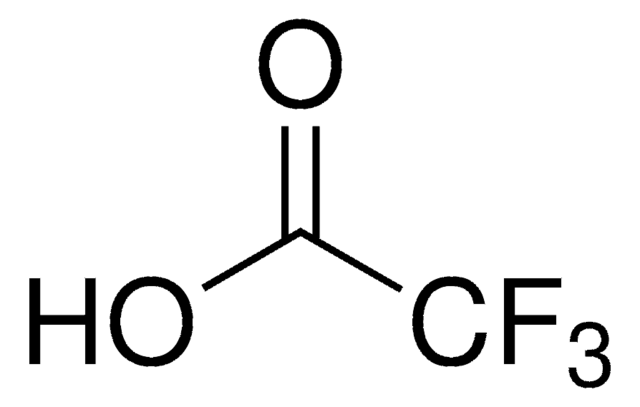
Trifluoromethanesulfonic acid
ReagentPlus®, ≥99%
Synonym(s):
Triflic acid
About This Item
Recommended Products
vapor density
5.2 (vs air)
Quality Level
vapor pressure
8 mmHg ( 25 °C)
product line
ReagentPlus®
Assay
≥99%
form
liquid
refractive index
n20/D 1.327 (lit.)
bp
162 °C (lit.)
density
1.696 g/mL at 25 °C (lit.)
SMILES string
OS(=O)(=O)C(F)(F)F
InChI
1S/CHF3O3S/c2-1(3,4)8(5,6)7/h(H,5,6,7)
InChI key
ITMCEJHCFYSIIV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Substituted tetrahydrofurans and tetrahydropyrans by cyclization of corresponding unsaturated alcohols under acidic conditions.
- Nitriles from corresponding aldehydes by Schmidt reaction.
- Disubstituted five-membered ring lactones by allylboration reaction between 2-alkoxycarbonyl allylboronates and aldehydes.
It can also be used as a catalyst in the Fischer glycosylation and Friedel-Crafts acylation reactions.
Legal Information
accessory
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Met. Corr. 1 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
>332.1 °F - Pensky-Martens closed cup
Flash Point(C)
> 166.7 °C - Pensky-Martens closed cup
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PDSCL
Deleterious substance
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
347817-1G-KC:
347817-100G:4548173310572
347817-5X1G:4548173138671
347817-1G:4548173138640
347817-5G:4548173138664
347817-BULK:
347817-VAR:
347817-500G:4548173321042
347817-5X1G-KC:
347817-25G:4548173138657
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service