U0750
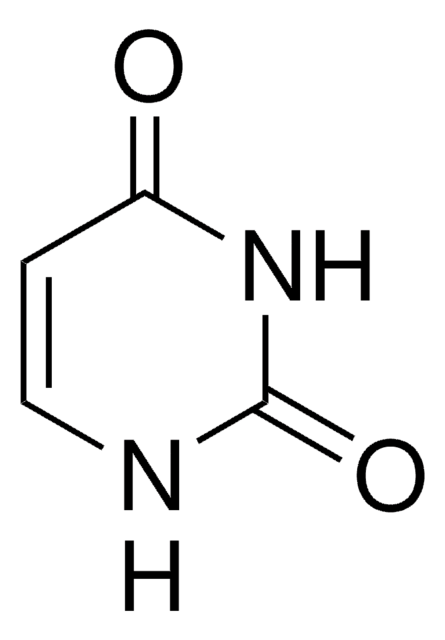
Uracil
≥99.0%
Sinonimo/i:
2,4(1H,3H)-Pyrimidinedione, 2,4-Dihydroxypyrimidine, 2,4-Pyrimidinediol
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C4H4N2O2
Numero CAS:
Peso molecolare:
112.09
Beilstein:
507828
Numero CE:
Numero MDL:
Codice UNSPSC:
41106305
ID PubChem:
NACRES:
NA.51
Prodotti consigliati
Origine biologica
synthetic (organic)
Livello qualitativo
Saggio
≥99.0%
Stato
powder
Punto di fusione
>300 °C (lit.)
Stringa SMILE
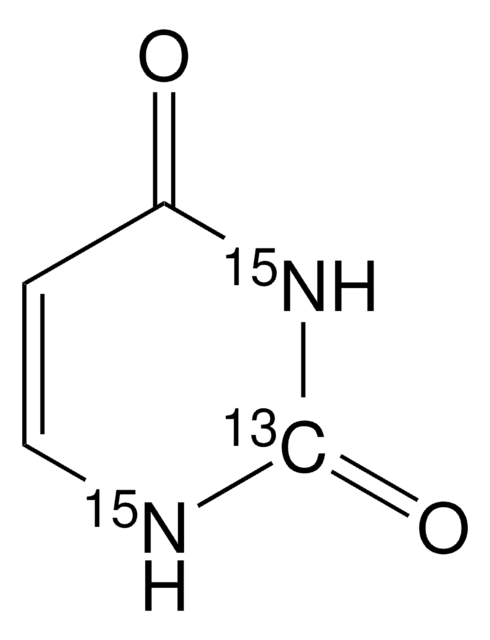
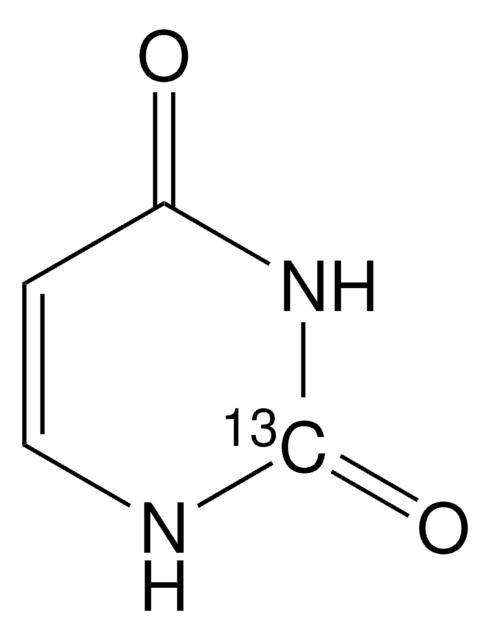
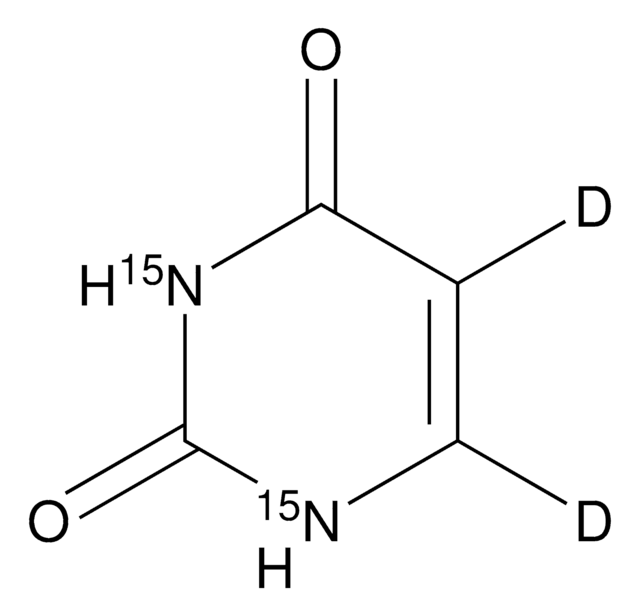
O=C1NC=CC(=O)N1
InChI
1S/C4H4N2O2/c7-3-1-2-5-4(8)6-3/h1-2H,(H2,5,6,7,8)
ISAKRJDGNUQOIC-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Uracil belongs to the pyrimidine nucleobase family. It is naturally occurring and is an important and active part of RNA.
Applicazioni
Uracil has been used as:
- a nucleotide standard to determine DNA methylation by high-performance liquid chromatography
- a component of synthetic define medium for culturing Saccharomyces cerevisiae and its strain
- a component of the amino acid supplement to culture Bacillus sp
Azioni biochim/fisiol
Uracil and its derivatives play an integral part in medicinal chemistry for the synthesis of cancer, viral infections, autosomal recessive disorder, thyroid, and diabetic drugs.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Sandra M Carvalho et al.
PloS one, 8(3), e58492-e58492 (2013-03-19)
Links between carbohydrate metabolism and virulence in Streptococcus pneumoniae have been recurrently established. To investigate these links further we developed a chemically defined medium (CDM) and standardized growth conditions that allowed for high growth yields of the related pneumococcal strains
William B White et al.
The New England journal of medicine, 369(14), 1327-1335 (2013-09-03)
To assess potentially elevated cardiovascular risk related to new antihyperglycemic drugs in patients with type 2 diabetes, regulatory agencies require a comprehensive evaluation of the cardiovascular safety profile of new antidiabetic therapies. We assessed cardiovascular outcomes with alogliptin, a new
Daisuke Katagiri et al.
Journal of the American Society of Nephrology : JASN, 24(12), 2034-2043 (2013-10-05)
Accumulating evidence of the beyond-glucose lowering effects of a gut-released hormone, glucagon-like peptide-1 (GLP-1), has been reported in the context of remote organ connections of the cardiovascular system. Specifically, GLP-1 appears to prevent apoptosis, and inhibition of dipeptidyl peptidase-4 (DPP-4)
Jaunius Urbonavicius et al.
Methods in enzymology, 425, 103-119 (2007-08-04)
Formation of 5-methyluridine (ribothymidine) at position 54 of the T-psi loop of tRNA is catalyzed by site-specific tRNA methyltransferases (tRNA[uracil-54,C5]-MTases). In eukaryotes and many bacteria, the methyl donor for this reaction is generally S-adenosyl-L-methionine (S-AdoMet). However, in other bacteria, like
Mirta M L Sousa et al.
Molecular aspects of medicine, 28(3-4), 276-306 (2007-06-26)
Uracil is usually an inappropriate base in DNA, but it is also a normal intermediate during somatic hypermutation (SHM) and class switch recombination (CSR) in adaptive immunity. In addition, uracil is introduced into retroviral DNA by the host as part
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.