T3405
3,3′,5,5′-Tetramethylbenzidine dihydrochloride
chromogenic, tablet
Sinonimo/i:
4,4′-Diamino-3,3′,5,5′-tetramethylbiphenyl dihydrochloride
About This Item
Prodotti consigliati
Nome del prodotto
3,3′,5,5′-Tetramethylbenzidine dihydrochloride, tablet, 1 mg substrate per tablet
Stato
tablet
Punto di fusione
≥300 °C
Temperatura di conservazione
2-8°C
Stringa SMILE
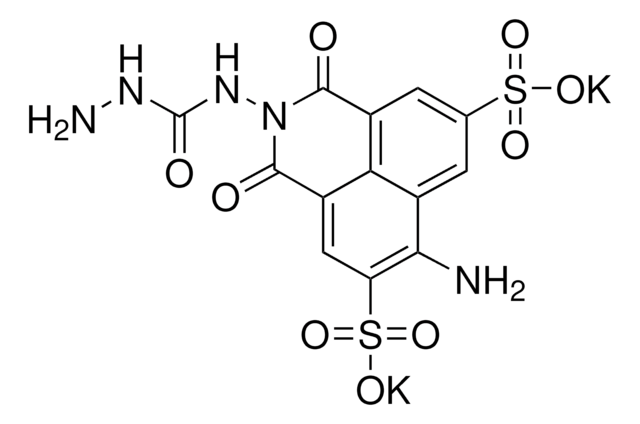
Cl[H].Cl[H].Cc1cc(cc(C)c1N)-c2cc(C)c(N)c(C)c2
InChI
1S/C16H20N2.2ClH/c1-9-5-13(6-10(2)15(9)17)14-7-11(3)16(18)12(4)8-14;;/h5-8H,17-18H2,1-4H3;2*1H
NYNRGZULARUZCC-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- anti-mouse IgG (? specific) horseradish peroxidase-conjugated antibody in the ELISA of mice sera
- anti-mouse peroxidase antibody in the protein tyrosine phosphatase assay of naive and effector T cell lysate
- in enzymatic hydroperoxide (EH) assay and in prooxidants–antioxidants balance (PAB) assay of serum samples from diabetic patients
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Nitroblue Tetrazolium (NBT) is used with the alkaline phosphatase substrate 5-Bromo- 4-Chloro-3-Indolyl Phosphate (BCIP) in western blotting and immunohistological staining procedures. These substrate systems produce an insoluble NBT diformazan end product that is blue to purple in color and can be observed visually.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.