ML0015
Aspartate Metabolite Library
Sinonimo/i:
Aspartic acid Metabolite Library
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Codice UNSPSC:
12352209
NACRES:
NA.25
Prodotti consigliati
Descrizione
Aspartate pathway
Livello qualitativo
Stato
solid
applicazioni
metabolomics
Temperatura di conservazione
−20°C
Descrizione generale
Aspartic acid (or aspartate) is a non-essential amino acid, which means that it is naturally synthesized by mammals. Aspartate presents many biochemical roles:
In the L-conformation, aspartic acid is a building block in the production of proteins, as well as aiding in many bodily functions, including the urea cycle, gluconeogenesis, and Krebs Cycle, a process that generates adenosine triphosphate (ATP). Aspartic acid also works as a neurotransmitter. The D-Aspartate conformation is linked to neurogenesis and endocrine systems.
In the L-conformation, aspartic acid is a building block in the production of proteins, as well as aiding in many bodily functions, including the urea cycle, gluconeogenesis, and Krebs Cycle, a process that generates adenosine triphosphate (ATP). Aspartic acid also works as a neurotransmitter. The D-Aspartate conformation is linked to neurogenesis and endocrine systems.
Applicazioni
The Aspartate Metabolite Library is a kit that contains a selection of 23 metabolite involved in Aspartate metabolism.These may be used for general research, as reagents or as reference compounds in analytical procedures.
Azioni biochim/fisiol
Aspartate roles and metabolites:
- Aspartate is synthesized by transamination of oxaloacetate through the actions of Aspartate aminotransferase and pyridoxal 5′- phosphate. Aspartyl-tRNA synthase can then couple the aspartate to aspartyl tRNA for protein synthesis.
- Aspartate carries the reducing equivalents in the mitochondrial Malate-Aspartate shuttle, which uses the ready interconversion of aspartate and oxaloacetate.
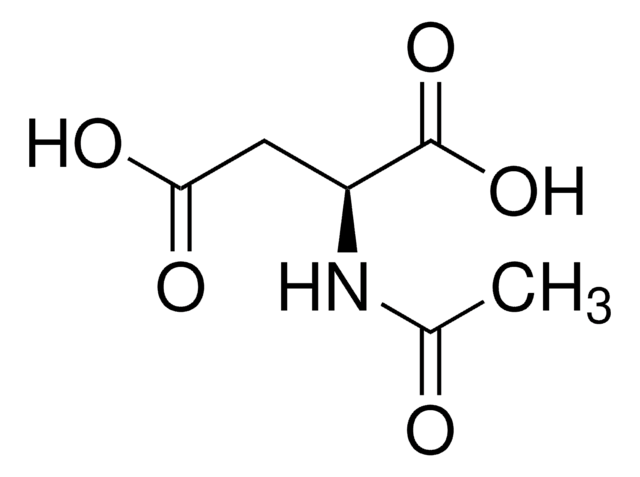
- N-acetylaspartate synthase, present in the cytoplasm, converts aspartate to N-acetylaspartate, a brain metabolite that regulates dopamine.
- Asparagine is biosynthesized by Asparagine synthetase from aspartate, glutamine, and ATP. Asparagine is involved in the metabolic control of cell functions in nerve and brain tissue.
- Arginosuccinic acid is synthesized from aspartate, citrulline and ATP through the action of Argininosuccinate synthase, one of the enzymes of the urea cycle. In this metabolic pathway, neurotoxic ammonia, produced by protein catabolism, is converted into urea in the liver.
- Fumaric acid is synthesized from Argininosuccinic acid via an Argininosuccinate lyase, which is an enzyme in the Citric Acid Cycle.
- Inosinic acid, aspartic acid and GTP are interconverted to GDP and AMP by the Adenylosuccinate synthetase isozyme 1. This process is involved in the purine nucleotide cycle which regulates nucleotides levels in various tissues.
- Aspartate transcarbamoylase catalyzes the synthesis of N-carbamoyl-L-aspartate from carbamoyl phosphate and aspartate that are involved in the de novo biosynthesis of pyrimidines.
- Beta alanine is formed by decarboxylation of aspartate by Glutamate decarboxylase 1 in the cytoplasm.
- L-aspartate is converted to D-aspartate through the action of a D-aspartate racemase. D-aspartate contributes to the synthesis and release of glucocorticoids, prolactin, oxytocin, and steroids. D-aspartate plays an important role in the brain activity of mammals.
Componenti
Contains 10 mg each of Aspartate metabolism metabolite standards packaged individually.
I componenti del kit sono disponibili anche separatamente
N° Catalogo
Descrizione
SDS
- A2252Adenosine 5′-monophosphate monohydrate, from yeast, ≥97%SDS
- A2383Adenosine 5′-triphosphate disodium salt hydrate, Grade I, ≥99%, from microbialSDS
- A5707Argininosuccinic acid disodium salt hydrate, ≥80%SDS
- 146064β-Alanine, 99%SDS
- C4135Carbamyl phosphate disodium salt, ≥80%SDS
- C7629L-Citrulline, ≥98% (TLC)SDS
- 219096D-Aspartic acid, ReagentPlus®, 99%SDS
- F6625Flavin adenine dinucleotide disodium salt hydrate, ≥95% (HPLC), powderSDS
- 47910Fumaric acid, ≥99.0% (T)SDS
- G7252Guanosine 5′-diphosphate tris salt from Saccharomyces cerevisiae, Type VI, ≥92.5%SDS
- G9002Guanosine 5′-triphosphate tris salt, ≥93% (HPLC), powderSDS
- I2879Inosine 5′-monophosphate from Saccharomyces cerevisiae, ≥98%SDS
- A5006L-Arginine, reagent grade, ≥98%SDS
- A0884L-Asparagine, ≥98% (HPLC)SDS
- 11189L-Aspartic acid, BioUltra, ≥99.5% (T)SDS
- G1251L-Glutamic acid, ReagentPlus®, ≥99% (HPLC)SDS
- G3126L-Glutamine, ReagentPlus®, ≥99% (HPLC)SDS
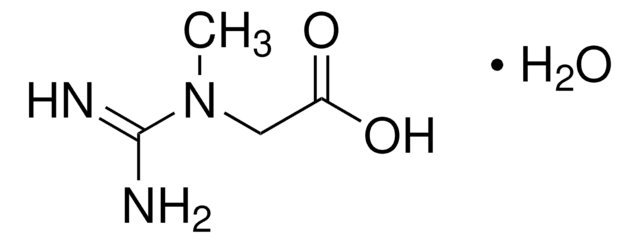
- 00920N-Acetyl-L-aspartic acid, ≥99.0% (T)SDS
- O4126Oxaloacetic acid, ≥97% (HPLC)SDS
- K1750α-Ketoglutaric acid, ≥98.5% (NaOH, titration)SDS
- P9255Pyridoxal 5′-phosphate hydrate, ≥98%SDS
- P8010Sodium pyrophosphate tetrabasic, ≥95%SDS
- 69037Ureidosuccinic acid, 98.0-102.0% (T)SDS
Vedi tutto (23)
Prodotti correlati
N° Catalogo
Descrizione
Determinazione del prezzo
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Dam. 1
Codice della classe di stoccaggio
11 - Combustible Solids
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Lot/Batch Number
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documenti section.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Pyrimidine Biosynthesis
Lennarz W J, et al.
Encyclopedia of Biological Chemistry, 600-605 (2004)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.