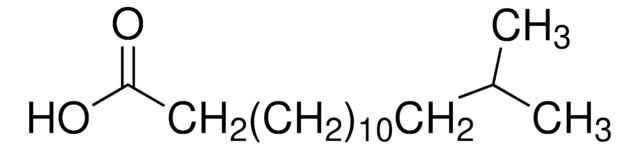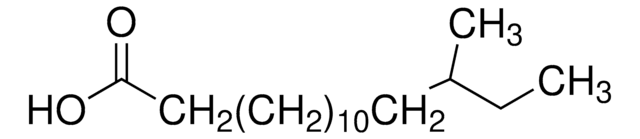M3664
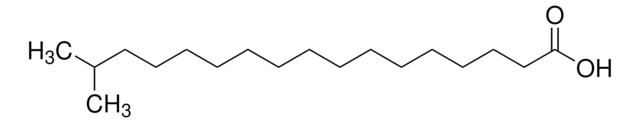
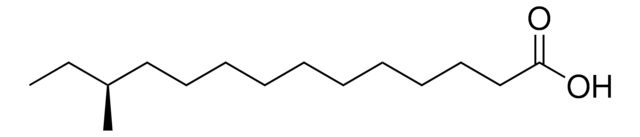
12-Methyltetradecanoic acid
Sinonimo/i:
(+)-12-Methyl myristic acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C15H30O2
Numero CAS:
Peso molecolare:
242.40
Numero MDL:
Codice UNSPSC:
12352211
ID PubChem:
NACRES:
NA.25
Prodotti consigliati
Saggio
≥98% (GC)
Livello qualitativo
Gruppo funzionale
carboxylic acid
Tipo di lipide
saturated FAs
Condizioni di spedizione
ambient
Temperatura di conservazione
2-8°C
Stringa SMILE
CCC(C)CCCCCCCCCCC(O)=O
InChI
1S/C15H30O2/c1-3-14(2)12-10-8-6-4-5-7-9-11-13-15(16)17/h14H,3-13H2,1-2H3,(H,16,17)
XKLJLHAPJBUBNL-UHFFFAOYSA-N
Azioni biochim/fisiol
12-Methyltetradecanoic acid (12-methylmyristic acid) is an anteiso-C(15)-fatty acid used in studies on the properties and functions of medium-chain anteiso-fatty acids. 12-methyltetradecanoic acid is studied to understand its antibacterial properties. 12-methyltetradecanoic acid represses the extracellular production of surfactants required for swarming motility in Pseudomonas aeruginosa PAO1.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
T Kitahara et al.
Bioscience, biotechnology, and biochemistry, 59(1), 78-82 (1995-01-01)
Both the enantiomers of aseanostatin P5 (sarcinic acid), an inhibitor of myeloperoxidase (MPO) release from human polymorphonuclear leukocytes (PMN), with high optical purity were synthesized by starting from (S)-2-methylbutanol and methyl (S)-3-hydroxy-2-methylpropanoate. They were converted to four diastereomers of aggreceride
M R Edgcomb et al.
Biochimica et biophysica acta, 1463(1), 31-42 (2000-01-13)
Listeria monocytogenes is a foodborne psychrotrophic pathogen that grows at refrigeration temperatures. Previous studies of fatty acid profiles of wild-type and cold-sensitive, branched-chain fatty acid deficient mutants of L. monocytogenes suggest that the fatty acid 12-methyltetradecanoic (anteiso-C(15:0)) plays a critical
Kenneth C Wright et al.
Journal of experimental therapeutics & oncology, 5(1), 55-68 (2006-01-19)
The purpose of this research was to evaluate the effects of targeted arterial delivery of the branched chain fatty acid 12-methyltetradecanoic acid (12-MTA) on the VX2 squamous cell carcinoma in rabbits. An intramuscular VX2 squamous cell carcinoma was induced at
Ying Xu et al.
Marine biotechnology (New York, N.Y.), 11(4), 495-504 (2008-11-26)
Eleven strains of Streptomyces isolated from deep-sea sediments were screened for anti-larval settlement activity and all were active. Among those strains, Streptomyces sp. UST040711-290 was chosen for the isolation of bioactive antifouling compounds through bioassay-guided isolation procedure. A branched-chain fatty
Ana Valéria Colnaghi Simionato et al.
Journal of mass spectrometry : JMS, 42(4), 490-496 (2007-02-14)
Xylella fastidiosa (X.f.) is a plant pathogen with high levels of genomic similarity to Xanthomonas campestris pv. campestris (X.c.c.). It has been shown that X. fastidiosa synthesizes a putative diffusible signal factor (X.f.-DSF) that activates regulation of pathogenicity factor (rpf)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.