L6170
β-Lactamase
recombinant, expressed in E. coli
Sinonimo/i:
Carbapenemase, Cephalosprinase
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Prodotti consigliati
Ricombinante
expressed in E. coli
Livello qualitativo
Forma fisica
powder
Attività specifica
≥20 U/mg (with cephalosporin C)
≥400 U/mg (with benzylpenicilin)
Temperatura di conservazione
2-8°C
Descrizione generale
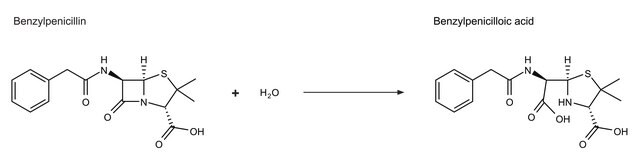
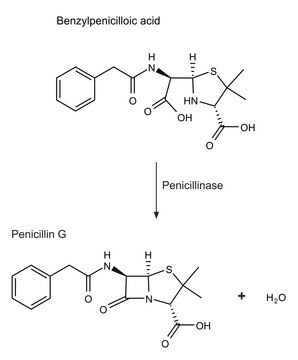
β-Lactamase produced by bacteria is closely related to the penicillin binding proteins. β-Lactamase hydrolyzes β-lactum antibiotics and is the prime cause of resistance development by bacteria. There are four subclasses of β-Lactamases. Classes A, C and D form an acyl-enzyme via active site serine residue. Class B β-lactamases are metalloenzymes with zinc ion at their active site for β-lactam hydrolysis. Mutations in the β-lactamases has resulted in the generation of extended-spectrum β-lactamases (ESBLs). As close to 900 types of β-Lactamases are produced by microbes.
Applicazioni
β--lactamase is used to inactivate β-lactam antibiotics by breaking open the β-lactam ring. β--lactamase is used to study antibiotic resistance and resistance suppression. Product L6170 is recombinantly produced based on the sequence of the enzyme from Pseudomonas aeruginosa, and is expressed in E. coli.
β-Lactamase has been used in coumarin-cephalosporin-fluorescein-acetoxymethyl (CCF4)- β-Lactamase assay in dendritic cells and in the hydrolysis of meropenem (H-Mer) to generate 18O-labeled H-Mer.
Azioni biochim/fisiol
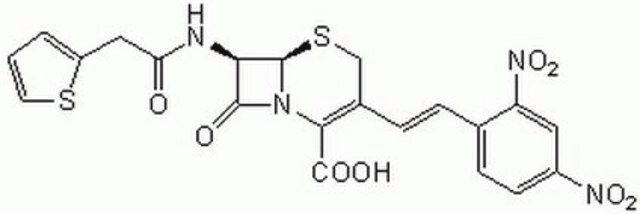
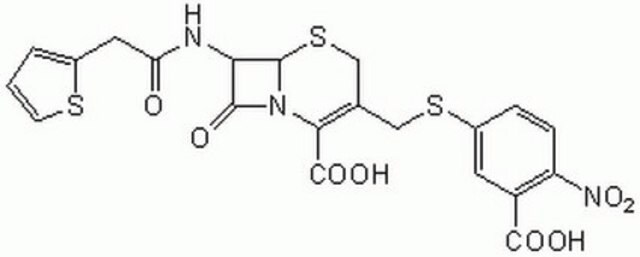
β--lactamase inactivates β-lactam antibiotics by breaking open the β-lactam ring. Typical analysis for other substrates: ceftriaxon 2-4 un/mg; cefazolin 2-4 un/mg; ceftazidime 1.5-3 un/mg; cefoxitin 0.35-0.7 un/mg; cefepime 1.2-2.4 un/mg; cefuroxime 1.5-3 un/mg; cefotaxime 1-2 un/mg, oxacillin 4-8 un/mg; imipenem 1.2-2.4 un/mg; and meropenem 3-6 un/mg
Typical analysis for other substrates: ceftriaxon 2-4 un/mg; cefazolin 2-4 un/mg; ceftazidime 1.5-3 un/mg; cefoxitin 0.35-0.7 un/mg; cefepime 1.2-2.4 un/mg; cefuroxime 1.5-3 un/mg; cefotaxime 1-2 un/mg, oxacillin 4-8 un/mg; imipenem 1.2-2.4 un/mg; and meropenem 3-6 un/mg
Definizione di unità
One unit will hydrolyze 1.0 μmole substrate per min (βI: benzylpenicillin; βII: cephalosporin C) in 50 mM phosphate buffer, pH 7.0 containing 10 μM zinc chloride at 25 ºC.
Stato fisico
Lyophilized powder containing sodium chloride and potassium phosphate.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
VAMP8-mediated NOX2 recruitment to endosomes is necessary for antigen release
Dingjan I, et al.
European Journal of Cell Biology, 96(7), 705-714 (2017)
Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment
Shaikh S, et al.
Saudi Journal of Biological Sciences, 22(1), 90-101 (2015)
Determining carbapenemase activity with 18O labeling and targeted mass spectrometry
Wang M, et al.
Analytical Chemistry, 85(22), 11014-11019 (2013)
The Growing Genetic and Functional Diversity of Extended Spectrum Beta-Lactamases
Ali T, et al.
BioMed Research International, 2018 (2018)
Updated functional classification of beta-lactamases
Bush K and Jacoby GA
Antimicrobial Agents and Chemotherapy, 54(3), 969-976 (2010)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.