About This Item
Formula empirica (notazione di Hill):
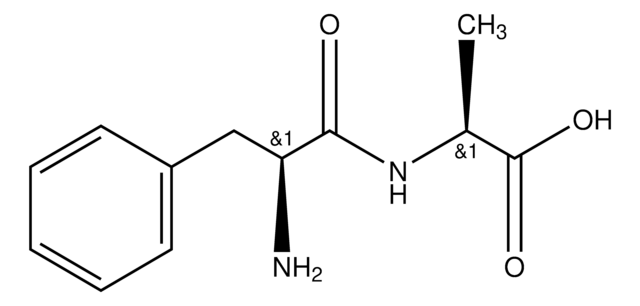
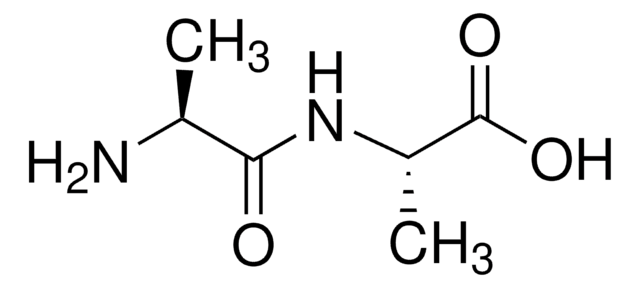
C12H24N2O3
Numero CAS:
Peso molecolare:
244.33
Numero CE:
Numero MDL:
Codice UNSPSC:
12352200
ID PubChem:
NACRES:
NA.26
Prodotti consigliati
Saggio
>98% (TLC)
Livello qualitativo
Stato
powder
tecniche
ligand binding assay: suitable
Colore
white to off-white
Temperatura di conservazione
−20°C
Stringa SMILE
CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(O)=O
InChI
1S/C12H24N2O3/c1-7(2)5-9(13)11(15)14-10(12(16)17)6-8(3)4/h7-10H,5-6,13H2,1-4H3,(H,14,15)(H,16,17)/t9-,10-/m0/s1
LCPYQJIKPJDLLB-UWVGGRQHSA-N
Categorie correlate
Amino Acid Sequence
Leu-Leu
Azioni biochim/fisiol
Leucylleucine (Leu-Leu) may be used to study the functionality of dileucine motifs such as the motif responsible for internalization and targeting of vesicular acetylcholine transporter and clathrin adaptors AP-1 and AP-2.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Lingling Zhang et al.
Oncogene, 39(49), 7196-7208 (2020-10-11)
Metastasis is responsible for the death of most breast cancer patients. Robo1 has been implicated as a tumor suppressor for various cancers including breast cancer. However, it is not well understood how Robo1 expression is regulated during tumorigenesis. In this
A van Boven et al.
Biochimie, 70(4), 535-542 (1988-04-01)
Different strains of Lactococcus lactis ssp. cremoris hydrolyze peptides at different rates while the cell-free extracts of these strains all show the same or much higher rates of hydrolysis. These observations indicate that the uptake of peptides is the rate-limiting
Yanzhen Zhou et al.
Journal of neuroinflammation, 15(1), 161-161 (2018-05-29)
Microglia-mediated neuroinflammation is recognized to mainly contribute to the pathogenesis of Parkinson's disease (PD). Tetrahydroxystilbene glucoside (TSG) has been proved to be beneficial for health with a great number of pharmacological properties. We examined the effects of TSG against dopamine
Pim W J M Frederix et al.
Dalton transactions (Cambridge, England : 2003), 41(42), 13112-13119 (2012-09-20)
A [FeFe]-hydrogenase model compound (µ-S(CH(2))(3)S)Fe(2)(CO)(4)(PMe(3))(2) [1] has been encapsulated in a low molecular weight (LMW) hydrogelator (Fmoc-Leu-Leu). Linear infrared absorption spectroscopy, gel melting and ultrafast time-resolved infrared spectroscopy experiments reveal significant contrasts in chemical environment and photochemistry between the encapsulated
Yayoi Miyashita et al.
Journal of cell science, 120(Pt 24), 4395-4406 (2007-12-07)
The E-cadherin-catenin complex regulates Ca(2+)-dependent cell-cell adhesion and is localized to the basolateral membrane of polarized epithelial cells. Uncoupling beta-catenin from E-cadherin by deletion or substitution mutations causes accumulation of these proteins in intracellular compartments, including the trans-Golgi network and
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.