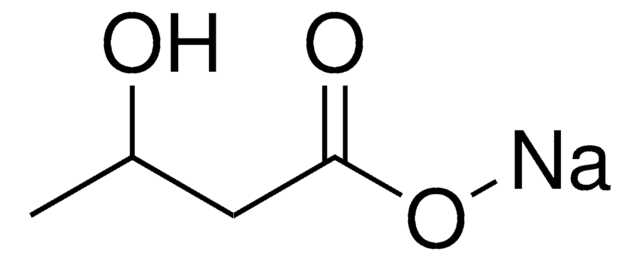
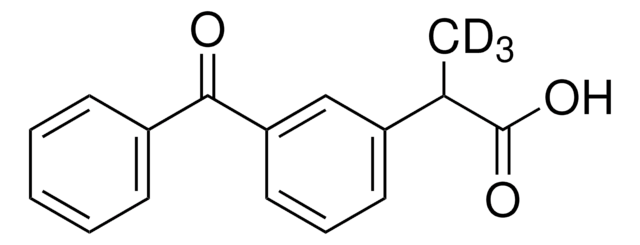
K1751
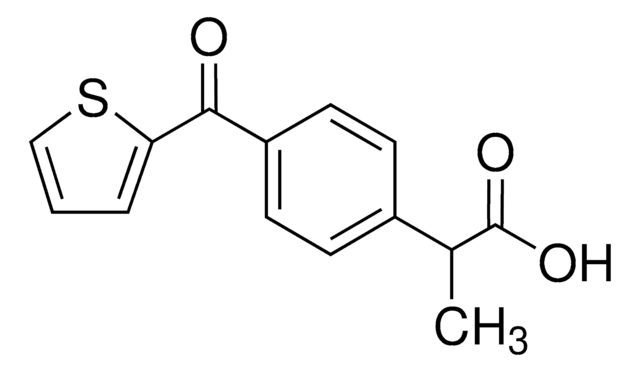
Ketoprofen
≥98% (TLC), powder, non-steroidal anti-inflammatory compound
Sinonimo/i:
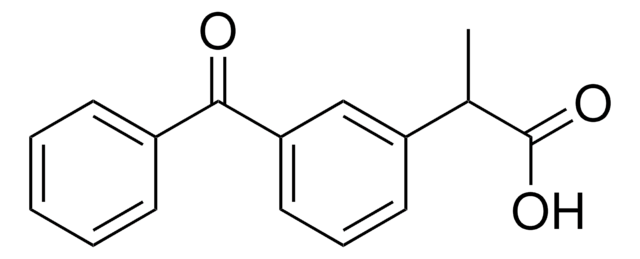
2-(3-Benzoylphenyl)propionic acid
About This Item
Prodotti consigliati
Nome del prodotto
Ketoprofen, ≥98% (TLC)
Origine biologica
synthetic
Saggio
≥98% (TLC)
Stato
powder
Solubilità
ethanol: 50 mg/mL, clear, colorless to yellow
Ideatore
Bayer
Stringa SMILE
CC(C(O)=O)c1cccc(c1)C(=O)c2ccccc2
InChI
1S/C16H14O3/c1-11(16(18)19)13-8-5-9-14(10-13)15(17)12-6-3-2-4-7-12/h2-11H,1H3,(H,18,19)
DKYWVDODHFEZIM-UHFFFAOYSA-N
Informazioni sul gene
human ... ALB(213) , IL8RA(3577) , PTGS1(5742) , PTGS2(5743)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- as sample to analyse the effects of long storage period by chromatographic and microscopic techniques
- as a nonselective COX inhibitor to inject subcutaneously in rats to study its effect on stress/methamphetamine hydrochloride-induced changes in occludin, claudin-5 and COX-2 protein immunoreactivity, truncation of β-dystroglycan, brain water content and fluorescein isothiocyanate-dextran extravasation
- in phosphate buffer to study its ability to inhibit heat-induced denaturation of albumin
Azioni biochim/fisiol
Caratteristiche e vantaggi
Applicazioni
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Oral - Aquatic Acute 1 - Eye Irrit. 2 - Skin Irrit. 2
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Human epithelial intestinal colonic organoids can be used as an alternative to Caco-2 drug permeability assays for drug screening and compound toxicity testing.
Chromatograms
application for HPLCapplication for HPLCapplication for HPLCIl team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.