H7021
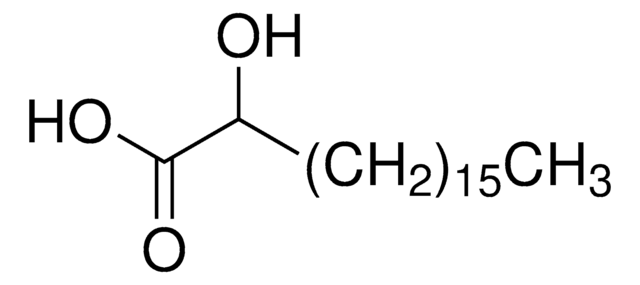
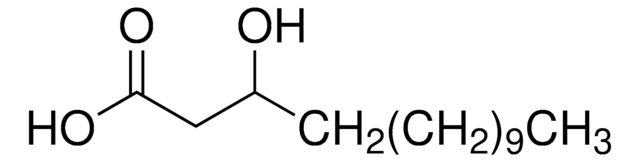
2-Hydroxyhexadecanoic acid
≥98% (capillary GC)
Sinonimo/i:
2-Hydroxypalmitic acid, DL-α-Hydroxypalmitic acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
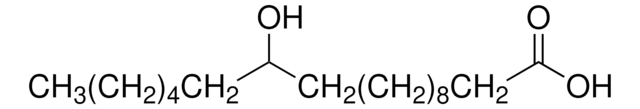
Formula condensata:
CH3(CH2)13CH(OH)COOH
Numero CAS:
Peso molecolare:
272.42
Numero CE:
Numero MDL:
Codice UNSPSC:
12352211
ID PubChem:
NACRES:
NA.25
Prodotti consigliati
Livello qualitativo
Saggio
≥98% (capillary GC)
Tipo di lipide
saturated FAs
Temperatura di conservazione
2-8°C
Stringa SMILE
CCCCCCCCCCCCCCC(O)C(O)=O
InChI
1S/C16H32O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15(17)16(18)19/h15,17H,2-14H2,1H3,(H,18,19)
JGHSBPIZNUXPLA-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Azioni biochim/fisiol
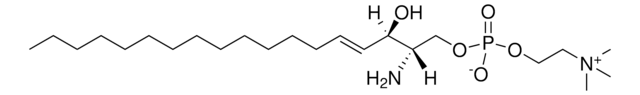
2-Hydroxyhexadecanoic acid (2OH-HDA) is used as a representative of the saturated long-chain hydroxyl fatty acids group, members of which have potential roles in anti-inflammatory action, neuroprotection, and bactericide and anti-cancer defense.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Elena E Pohl et al.
Biochimica et biophysica acta, 1778(5), 1292-1297 (2008-03-04)
Hydroxyl group-containing fatty acids play an important role in anti-inflammatory action, neuroprotection, bactericide and anti-cancer defense. However, the mechanism of long-chain hydroxy fatty acids (HFA) transport across plasma membranes is still disputed. Two main hypotheses have been suggested: firstly, that
Tomotake Morita et al.
Bioscience, biotechnology, and biochemistry, 75(8), 1597-1599 (2011-08-09)
Cryptococcus humicola JCM 1461 efficiently produced cellobiose lipids (CLs), bolaform biosurfactants. The main product was identified as 16-O-(2″,3″,4″,6'-tetra-O-acetyl-β-cellobiosyl)-2-hydroxyhexadecanoic acid. The production yield of CLs reached 13.1 g/L under the intermittent feeding of glucose. The critical micelle concentrations (CMC) of the
J A Hamilton
Prostaglandins, leukotrienes, and essential fatty acids, 60(5-6), 291-297 (1999-09-02)
In early research on fatty acid transport, passive diffusion seemed to provide an adequate explanation for movement of fatty acids through the membrane bilayer. This simple hypothesis was later challenged by the discovery of several proteins that appeared to be
T Kaneshiro et al.
Lipids, 28(5), 397-401 (1993-05-01)
Fumonisin B1 is a sphingolipid-like compound that enhances the accumulation of yeast sphingolipids and 2-hydroxy fatty acids. These lipids occur both as freely extractable and cell bound components in yeast fermentations. Both free and bound 2-hydroxy fatty acids produced by
S Hamanaka et al.
Biochimica et biophysica acta, 961(3), 374-377 (1988-08-12)
Monoglycosylceramides were isolated from pig epidermal cells which had been prepared free from dermal elements. The most polar glycolipid among the five isolated monoglycosylceramides was galactosylceramide. The galactosylceramide was composed of alpha-hydroxypalmitic acid and 16- and 18-carbon chain sphingenine, being
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.