H2775
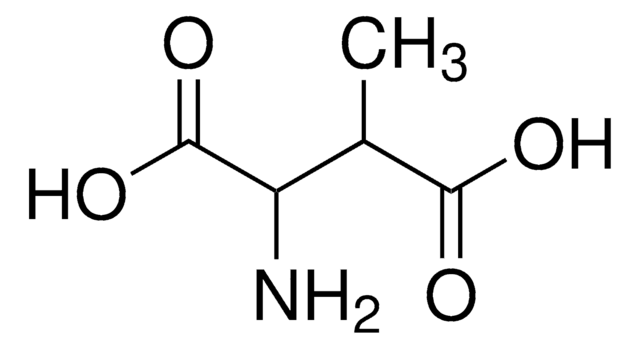
DL-threo-β-Hydroxyaspartic acid
Sinonimo/i:
threo-2-Amino-3-hydroxysuccinic acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C4H7NO5
Numero CAS:
Peso molecolare:
149.10
Numero CE:
Numero MDL:
Codice UNSPSC:
12352106
eCl@ss:
32160406
ID PubChem:
NACRES:
NA.32
Prodotti consigliati
Livello qualitativo
Temperatura di conservazione
−20°C
Stringa SMILE
N[C@H]([C@@H](O)C(O)=O)C(O)=O
InChI
1S/C4H7NO5/c5-1(3(7)8)2(6)4(9)10/h1-2,6H,5H2,(H,7,8)(H,9,10)/t1-,2-/m1/s1
YYLQUHNPNCGKJQ-JCYAYHJZSA-N
Informazioni sul gene
human ... GLUL(2752)
mouse ... GLUL(14645)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
DL-threo-β-hydroxyaspartic acid (THA) is a glutamate uptake inhibitor.
Applicazioni
DL-threo-β-Hydroxyaspartic acid (THA) has been used to block glutamate transport in cannulated sprague dawley rat.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Nina Bionda et al.
Amino acids, 42(1), 285-293 (2010-11-18)
A simple and practical general synthetic protocol towards orthogonally protected tHyAsp derivatives fully compatible with Fmoc solid-phase peptide synthetic methodology is reported. Our approach includes enantioresolution of commercially available D: ,L: -tHyAsp racemic mixture by co-crystallization with L: -Lys, followed
Ewa Nagańska et al.
Folia neuropathologica, 48(1), 35-44 (2010-04-13)
Erythropoietin (EPO) is a chemokine hormone that is widely distributed throughout the body including nervous system. For last years its role as cytokine involved in many physiological processes out of the bone marrow has been suggested. Moreover, it plays a
Claudio Laurido et al.
TheScientificWorldJournal, 2012, 279147-279147 (2012-04-27)
N-methyl-D-aspartic acid receptor (NMDAr) activation requires the presence of D-serine, synthesized from L-serine by a pyridoxal 5'-phosphate-dependent serine racemase (SR). D-serine levels can be lowered by inhibiting the racemization of L-serine. L-serine-O-sulfate (LSOS) and L-erythro-3-hydroxyaspartate (LEHA), among others, have proven
A Hirata et al.
Brain research, 771(1), 37-44 (1998-02-12)
Excitotoxicity secondary to the loss of glutamate transporters (GluT) has been proposed as a possible pathogenetic mechanism for neuronal degeneration in amyotrophic lateral sclerosis. We therefore investigated whether prolonged in vivo pharmacologic inhibition of GluT would result in neuronal damage
A Klegeris et al.
Journal of neuroimmunology, 78(1-2), 152-161 (1997-10-23)
Glutamate, an excitatory neurotransmitter, is neurotoxic at high concentrations. Neuroglial cells, including astrocytes and microglia, play an important role in regulating its extracellular levels. Cultured human monocytic THP-1 cells increased their glutamate secretion following 18 and 68 h exposure to
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.