G9753
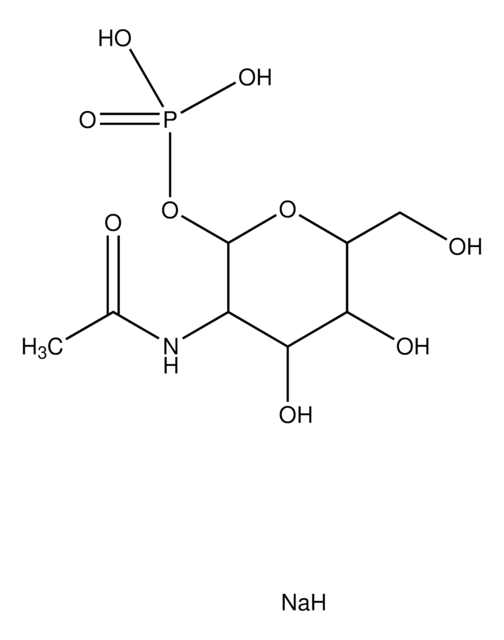
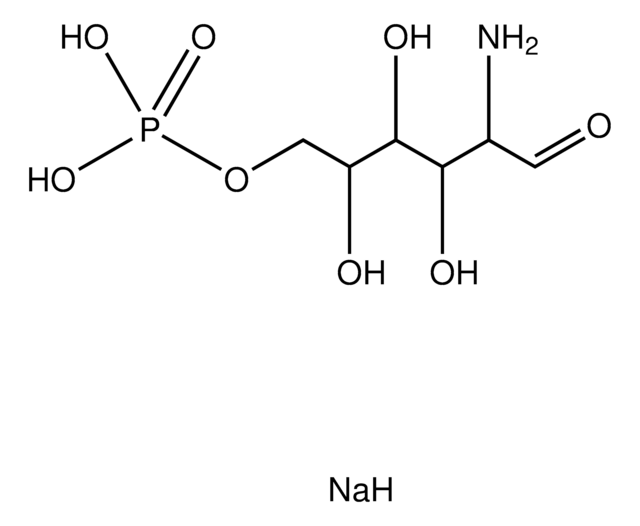
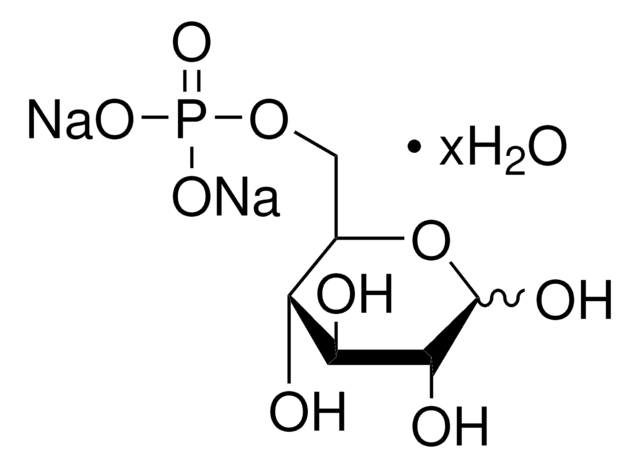
α-D-Glucosamine 1-phosphate
Sinonimo/i:
2-Amino-2-deoxy-α-D-glucopyranosyl phosphate
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C6H14NO8P
Numero CAS:
Peso molecolare:
259.15
Numero MDL:
Codice UNSPSC:
12352201
ID PubChem:
NACRES:
NA.25
Prodotti consigliati
Origine biologica
natural (inorganic)
Livello qualitativo
Saggio
≥97% (TLC)
Stato
powder
Impurezze
<8.5% water (Karl Fischer)
Colore
white
Solubilità
water: 100 mg/mL, clear, colorless
Temperatura di conservazione
−20°C
Stringa SMILE
NC1C(O)C(O)C(CO)OC1OP(O)(O)=O
InChI
1S/C6H14NO8P/c7-3-5(10)4(9)2(1-8)14-6(3)15-16(11,12)13/h2-6,8-10H,1,7H2,(H2,11,12,13)
YMJBYRVFGYXULK-UHFFFAOYSA-N
Categorie correlate
Applicazioni
- Cellodextrin phosphorylase from Ruminiclostridium thermocellum: X-ray crystal structure and substrate specificity analysis. This study presents the enzymatic synthesis and analysis of alpha-ᴅ-Glucosamine 1-phosphate based polysaccharides using cellodextrin phosphorylase, showcasing potential for novel biomaterial development. Field et al., 2017
- Glucose-1-phosphate uridylyltransferase from Erwinia amylovora: Activity, structure and substrate specificity. This paper explores the biochemical pathway involving alpha-ᴅ-Glucosamine 1-phosphate in the context of bacterial metabolism, providing insights into microbial biochemistry and potential targets for antibacterial therapy. Field et al., 2017
Altre note
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
D Mengin-Lecreulx et al.
The Journal of biological chemistry, 271(1), 32-39 (1996-01-05)
Two different approaches to identify the gene encoding the phosphoglucosamine mutase in Escherichia coli were used: (i) the purification to near homogeneity of this enzyme from a wild type strain and the determination of its N-terminal amino acid sequence; (ii)
Fumitaka Kudo et al.
Journal of the American Chemical Society, 127(6), 1711-1718 (2005-02-11)
Aminoglycoside antibiotics are composed of aminosugars and a unique aminocyclitol aglycon including 2-deoxystreptamine (DOS), streptidine, actinamine, etc., and nucleotidylyltransferases, sugar modifying enzymes, and glycosyltransferases appear to be essential for their biosynthesis. However, the genes encoding those enzymes were unable to
E V Vorob'eva et al.
Bioorganicheskaia khimiia, 32(5), 538-545 (2006-10-18)
The hydrolysis of defatted cells of the marine bacterium Chryseobacterium scophtalmum CIP 104199T with 10% acetic acid (3 h, 100 degrees C) led to an unusual lipid A (LA) (yield 0.6%), obtained for the first time. Using chemical analysis, FAB
S Ambrosio et al.
Journal of biochemical and biophysical methods, 25(4), 237-244 (1992-12-01)
Galactosamine is quickly metabolized to galactosamine 1-phosphate in rats treated with this compound. An HPLC method to quantify hexosamine phosphates in biological samples is described, modified from the o-phthaldialdehyde amino acid analysis procedure. o-Phthaldialdehyde derivatives of hexosamines and hexosamine-phosphates can
Seema C Namboori et al.
Journal of bacteriology, 190(8), 2987-2996 (2008-02-12)
Archaea and eukaryotes share a dolichol phosphate-dependent system for protein N-glycosylation. In both domains, the acetamido sugar N-acetylglucosamine (GlcNAc) forms part of the core oligosaccharide. However, the archaeal Methanococcales produce GlcNAc using the bacterial biosynthetic pathway. Key enzymes in this
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.