F6892
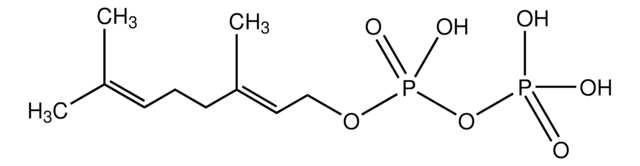
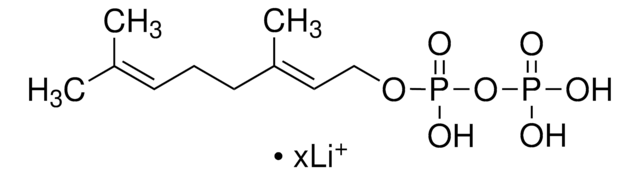
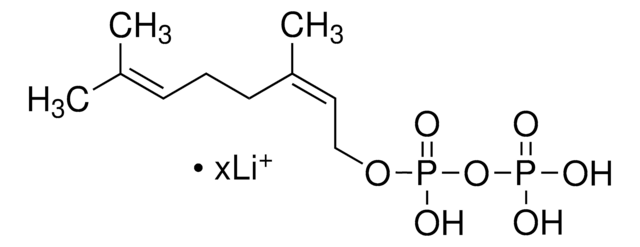
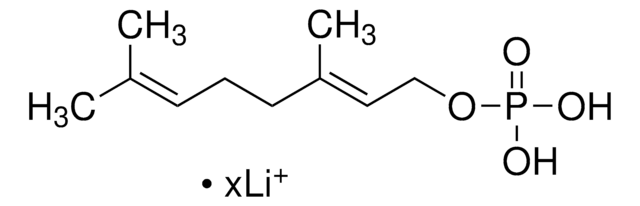
Farnesyl pyrophosphate ammonium salt
methanol:ammonia solution, ≥95% (TLC)
Sinonimo/i:
3,7,11-Trimethyl-2,6,10-dodecatrien-1-yl pyrophosphate ammonium salt, FPP
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥95% (TLC)
Stato
methanol:ammonia solution
Confezionamento
vial of 200 μg
Temperatura di conservazione
−20°C
Stringa SMILE
C\C(C)=C\CC\C(C)=C\CC\C(C)=C\COP(O)(=O)OP(O)(O)=O
InChI
1S/C15H28O7P2/c1-13(2)7-5-8-14(3)9-6-10-15(4)11-12-21-24(19,20)22-23(16,17)18/h7,9,11H,5-6,8,10,12H2,1-4H3,(H,19,20)(H2,16,17,18)/b14-9+,15-11+
VWFJDQUYCIWHTN-YFVJMOTDSA-N
Informazioni sul gene
rat ... Fnta(25318)
Descrizione generale
Applicazioni
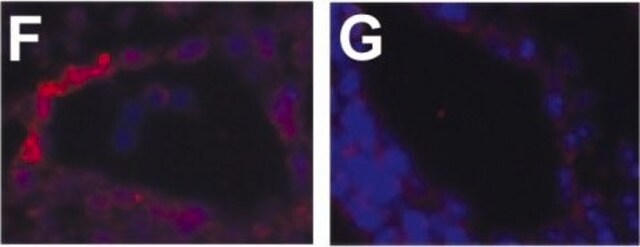
- as a prenylation agonist in human osteogenic sarcoma cells in collagen-based cell invasion assays
- in the prenylation of the hepatocyte growth factor (HGF) in human umbilical vein endothelial cells (HUVECs)
- as a substrate in prenyltransferases assay in diatom Haslea ostrearia
Azioni biochim/fisiol
Stato fisico
Actual concentration given on label
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 1
Organi bersaglio
Eyes,Central nervous system
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
60.8 °F
Punto d’infiammabilità (°C)
16 °C
Elenchi normativi
Forniamo informazioni su eventuali restrizioni prevalentemente per i prodotti chimici. Per altre tipologie di prodotto siamo in grado di fornire soltanto informazioni limitate. Nessuna segnalazione significa che nessuno dei componenti è citato in un elenco. È dovere dell’utilizzatore assicurarsi che il prodotto venga impiegato in maniera sicura e a norme di legge.
EU REACH Annex XVII (Restriction List)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Terpenes comprise the largest and most diverse class of secondary metabolites; approximately 55,000 compounds have been identified to date.
Biosynthesis of cholesterol generally takes place in the endoplasmic reticulum of hepatic cells and begins with acetyl- CoA, which is mainly derived from an oxidation reaction in the mitochondria. Acetyl-CoA and acetoacetyl-CoA are converted to 3-hydroxy- 3-methylglutaryl-CoA (HMG-CoA) by HMG-CoA synthase.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.