C1159
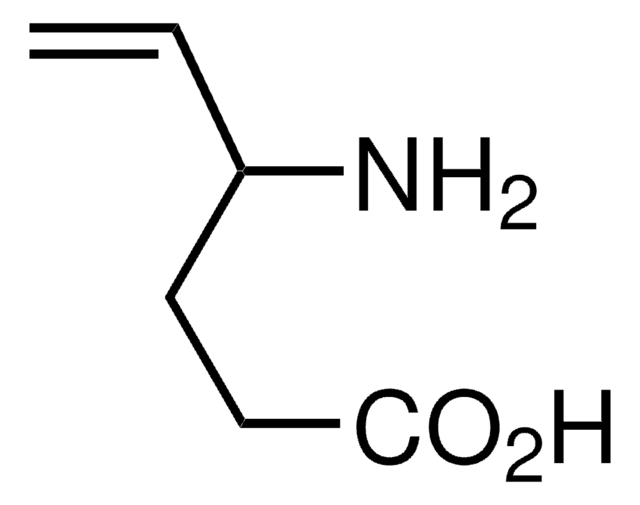
L-Cycloserine
Sinonimo/i:
(S)-4-Amino-3-isoxazolidone
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C3H6N2O2
Numero CAS:
Peso molecolare:
102.09
Beilstein:
80799
Numero CE:
Numero MDL:
Codice UNSPSC:
12352202
eCl@ss:
32160406
ID PubChem:
NACRES:
NA.77
Prodotti consigliati
Saggio
≥95% (TLC)
Livello qualitativo
Stato
powder
Punto di fusione
146 °C
Solubilità
H2O: 50 mg/mg protein
Temperatura di conservazione
−20°C
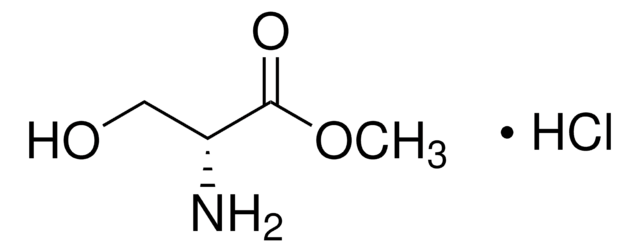
Stringa SMILE
N[C@H]1CONC1=O
InChI
1S/C3H6N2O2/c4-2-1-7-5-3(2)6/h2H,1,4H2,(H,5,6)/t2-/m0/s1
DYDCUQKUCUHJBH-REOHCLBHSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Azioni biochim/fisiol
Blocks sphingosine biosynthesis by inhibition of ketosphinganine synthetase. Cytotoxicity toward neuroblastoma and medulloblastoma cells mediated by suppression of ganglioside synthesis.
L-cycloserine is a potent inhibitor of serine palmitoyltransferase, the first step of sphingolipid synthesis.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Geraldine Rath et al.
The international journal of biochemistry & cell biology, 41(5), 1165-1172 (2008-11-26)
Doxorubicin and camptothecin are two cytotoxic chemotherapeutic agents triggering apoptosis in various cancer cells, including thyroid carcinoma cells. Recent studies revealed a critical role of ceramide in chemotherapy and suggested that anti-cancer drugs may kill tumor cells through sphingomyelinase activation.
David M Pereira et al.
Marine drugs, 12(1), 54-68 (2013-12-26)
We describe the effect of a chemically characterized lipophilic extract obtained from Marthasterias glacialis L. against human breast cancer (MCF-7) and human neuroblastoma (SH-SY5Y) cell lines. Evaluation of DNA synthesis revealed that both cell lines were markedly affected in a
Peirong Hu et al.
Journal of neuroscience research, 94(11), 1152-1168 (2016-09-18)
Currently, presymtomatic hematopoietic stem and progenitor cell transplantation (HSPCT) is the only therapeutic modality that alleviates Krabbe's disease (KD)-induced central nervous system damage. However, all HSPCT-treated patients exhibit severe deterioration in peripheral nervous system function characterized by major motor and
J Cinatl et al.
Anticancer research, 19(6B), 5349-5354 (2000-03-04)
Human neuroblastoma and medulloblastoma express abnormal ganglioside patterns especially GD2 and GM2 which are important for tumour growth. We tested the effects of L-cycloserine (L-CS), a potent inhibitor of synthesis of glycosphingolipids, on the growth, viability and expression of GD2
Karim Bennaceur et al.
Glycobiology, 19(6), 576-582 (2009-02-26)
Tumor escape is linked to multiple mechanisms, notably the liberation, by tumor cells, of soluble factors that inhibit the function of dendritic cells (DC). We have shown that melanoma gangliosides impair DC differentiation and induce their apoptosis. The present study
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.