C6880
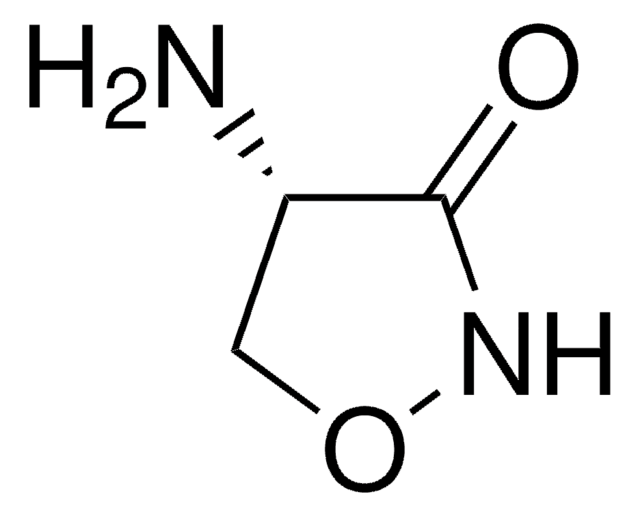
D-Cycloserine
Sinonimo/i:
(R)-4-Amino-3-isoxazolidone, 4-Amino-3-isoxazolidinone
About This Item
Prodotti consigliati
Forma fisica
powder
Punto di fusione
147 °C (dec.) (lit.)
Spettro attività antibiotica
Gram-negative bacteria
mycobacteria
Modalità d’azione
cell wall synthesis | interferes
Temperatura di conservazione
−20°C
Stringa SMILE
N[C@@H]1CONC1=O
InChI
1S/C3H6N2O2/c4-2-1-7-5-3(2)6/h2H,1,4H2,(H,5,6)/t2-/m1/s1
DYDCUQKUCUHJBH-UWTATZPHSA-N
Informazioni sul gene
human ... GRIN1(2902) , GRIN2A(2903) , GRIN2B(2904) , GRIN2C(2905) , GRIN2D(2906) , GRIN3A(116443) , GRIN3B(116444) , GRINA(2907)
mouse ... GRIN1(14810) , GRIN2A(14811) , GRIN2B(14812) , GRIN2C(14813) , GRIN2D(14814) , GRIN3A(242443) , GRIN3B(170483) , GRINA(66168)
rat ... GRIN1(24408) , GRIN2A(24409) , GRIN2B(24410) , GRIN2C(24411) , GRIN2D(24412) , GRIN3A(191573) , GRIN3B(170796) , GRINA(266668)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- to study its effect on partner preference in Prairie Voles
- to study its effect on grooming behaviour of rats
- to determine its minimum inhibitory concentration (MIC) on 48 multidrug resistant tuberculosis (MDR-TB) isolates using the broth microdilution method
- to study its effect on mouse model of autism, using behavioural assays
Azioni biochim/fisiol
Partial agonist at the glycine modulatory site of NMDA glutamatergic receptors; antibiotic against Gram-negative bacteria.
Mode of Resistance: D-Ala transport interference.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.