B8271
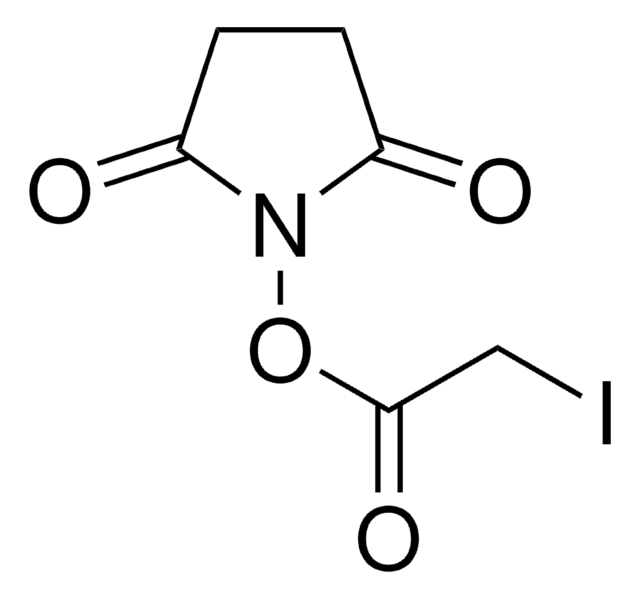
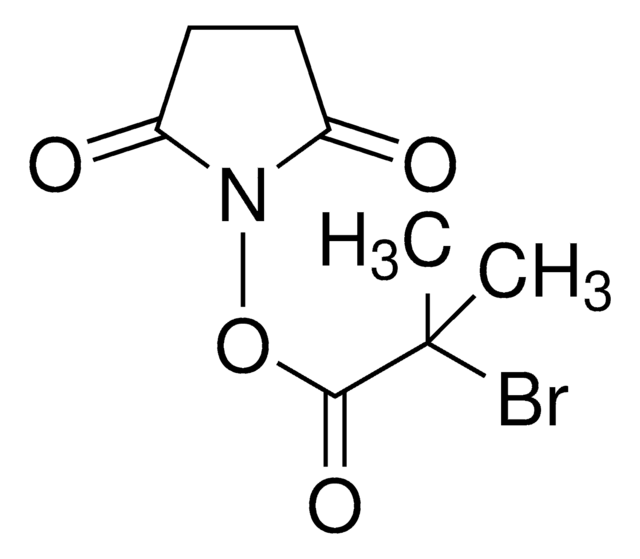
Bromoacetic acid N-hydroxysuccinimide ester
≥95%, powder
Sinonimo/i:
2,5-Dioxopyrrolidin-1-yl 2-bromoacetate, N-Hydroxysuccinimide bromoacetate
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C6H6BrNO4
Numero CAS:
Peso molecolare:
236.02
Numero MDL:
Codice UNSPSC:
12352106
ID PubChem:
NACRES:
NC.07
Prodotti consigliati
Livello qualitativo
Saggio
≥95%
Stato
powder
Impiego in reazioni chimiche
reagent type: cross-linking reagent
Solubilità
acetone: 25 mg/mL
DMF: soluble
Gruppo funzionale
NHS ester
Condizioni di spedizione
dry ice
Temperatura di conservazione
−20°C
Stringa SMILE
O=C(N1OC(CBr)=O)CCC1=O
InChI
1S/C6H6BrNO4/c7-3-6(11)12-8-4(9)1-2-5(8)10/h1-3H2
NKUZQMZWTZAPSN-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
A heterobifunctional cross-linking reagent which allows bromoacetylation of primary amine groups followed by coupling to sulfhydryl-containing compounds. Typically, initial reaction couples via ester to primary amine by amide bond formation in the pH range 6.5-8.5. The second reaction results in thioether bonding in pH range 7.0-8.0.
Avvertenza
The bromoacetyl group is light sensitive.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
N Kolodny et al.
Analytical biochemistry, 187(1), 136-140 (1990-05-15)
A method described here for conjugating synthetic peptides to carrier proteins provides a convenient method for determining peptide-to-carrier protein ratios. N-Bromoacetyl-containing peptides are reacted in situ with carrier proteins in which the disulfide bonds were reduced with tri-n-butylphosphine. At pH
M S Bernatowicz et al.
Analytical biochemistry, 155(1), 95-102 (1986-05-15)
Synthetic peptides derived from human fibrin were unidirectionally conjugated to three carrier proteins (bovine serum albumin, bovine alpha-lactalbumin, and keyhole limpet hemocyanin) by a method that employs N-succinimidyl bromoacetate. This heterobifunctional crosslinking reagent was prepared with a 79% yield in
John S Mort et al.
Methods in molecular medicine, 100, 237-250 (2004-07-29)
The use of synthetic peptides to generate rabbit polyclonal anticatabolic neoepitope antibodies that can be used to study the presence of defined proteolytic cleavage sites in aggrecan is described. Principles of peptide design and methods for preparation and characterization of
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.