A5502
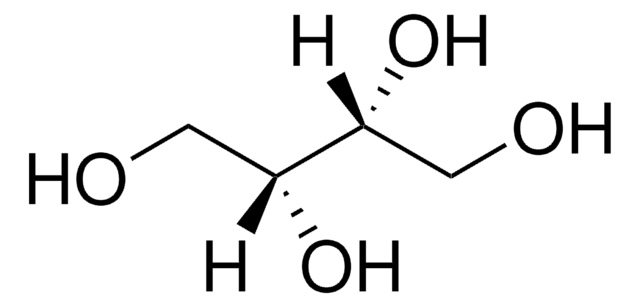
Adonitol
≥99%
Sinonimo/i:
Adonite, Ribitol
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C5H12O5
Numero CAS:
Peso molecolare:
152.15
Beilstein:
1720524
Numero CE:
Numero MDL:
Codice UNSPSC:
12352201
ID PubChem:
NACRES:
NA.25
Prodotti consigliati
Livello qualitativo
Saggio
≥99%
Stato
powder
Colore
white
Punto di fusione
104 °C ((219 °F ))
Solubilità
water: 50 mg/mL, clear to slightly hazy, colorless to faintly yellow
Stringa SMILE
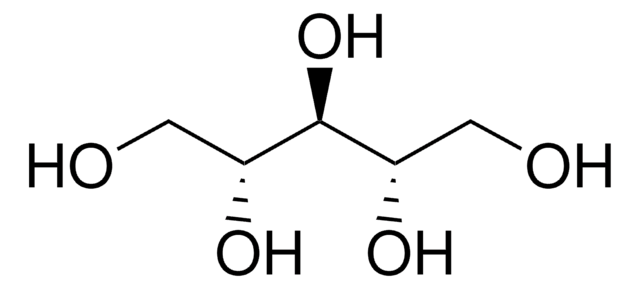
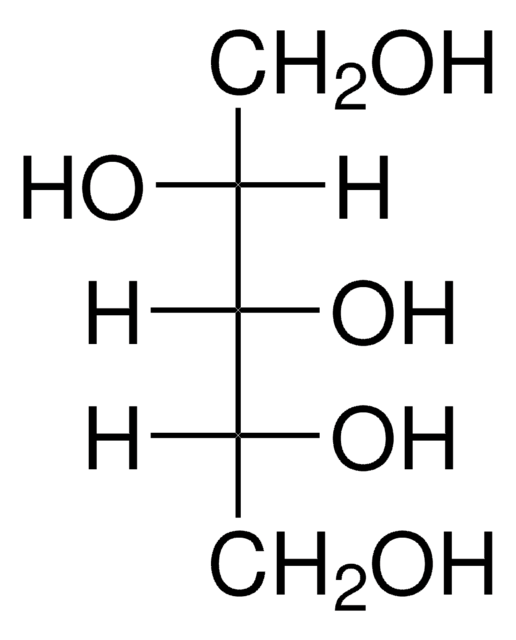
OC[C@H](O)[C@H](O)[C@H](O)CO
InChI
1S/C5H12O5/c6-1-3(8)5(10)4(9)2-7/h3-10H,1-2H2/t3-,4+,5-
HEBKCHPVOIAQTA-ZXFHETKHSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
Adonitol (Ribitol), a pentose alcohol, is metabolized to teicholic acids used in the cell walls of gram positive bacteria. Adonitol is often compared to other cell permeating molecules such as formamide, propanediol, and DMSO as a cryopreservation agent.
Altre note
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Anders Østergaard Madsen et al.
The journal of physical chemistry. A, 115(26), 7794-7804 (2011-06-15)
X-ray diffraction data of high quality measured to high resolution on crystals of the two pentitol epimers ribitol (centric) and xylitol (acentric) at 101, 141, and 181 K and data on the two compounds previously recorded at 122 K have
G Funke et al.
Journal of clinical microbiology, 31(11), 2907-2912 (1993-11-01)
Fifteen strains of CDC group 1 coryneform and biochemically similar bacteria were isolated from clinical specimens. Of the 15 strains isolated, 11 were derived from abscesses and purulent lesions, mostly from the upper part of the body, and 3 were
S Brisse et al.
Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases, 10(10), 942-945 (2004-09-18)
A rapid method combining gyrA PCR-restriction fragment length polymorphism analysis, parC PCR and adonitol fermentation was developed to identify Klebsiella pneumoniae phylogenetic groups KpI, KpII and KpIII. Analysis of 420 clinical isolates from 26 hospitals showed that the three groups
D J Brenner et al.
Journal of clinical microbiology, 15(4), 703-713 (1982-04-01)
DNA relatedness was used to define the biochemical boundaries of Escherichia coli. A large number of biochemically atypical strains were shown to belong to biogroups of E. coli. These included strains negative in reactions for indole, all three decarboxylases, D-mannitol
John Quiroga et al.
Frontiers in veterinary science, 8, 625347-625347 (2021-04-03)
Acute ruminal acidosis (ARA) occurs after an excessive intake of rapidly fermentable carbohydrates and is characterized by the overproduction of D-lactate in the rumen that reaches the bloodstream. Lameness presentation, one of the primary consequences of ARA in cattle, is
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.