A0783
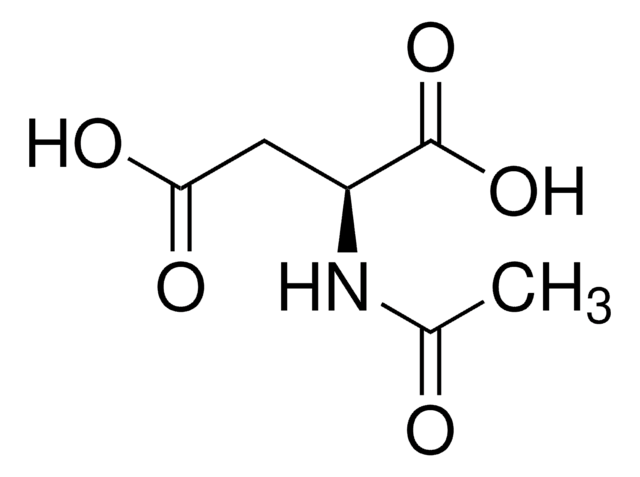
N-Acetyl-L-proline
≥98% (TLC), suitable for ligand binding assays
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C7H11NO3
Numero CAS:
Peso molecolare:
157.17
Numero CE:
Numero MDL:
Codice UNSPSC:
12352209
eCl@ss:
32160406
ID PubChem:
NACRES:
NA.26
Prodotti consigliati
Nome del prodotto
N-Acetyl-L-proline,
Saggio
≥98% (TLC)
Livello qualitativo
Stato
powder
tecniche
ligand binding assay: suitable
Colore
white
Temperatura di conservazione
2-8°C
Stringa SMILE
CC(=O)N1CCC[C@H]1C(O)=O
InChI
1S/C7H11NO3/c1-5(9)8-4-2-3-6(8)7(10)11/h6H,2-4H2,1H3,(H,10,11)/t6-/m0/s1
GNMSLDIYJOSUSW-LURJTMIESA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Azioni biochim/fisiol
N-Acetyl-L-proline, an analog of the COOH-terminal dipeptide portion of preferred angiotensin-converting enzyme substrates, is use to probe the active site of angiotensin-converting enzyme(s). N-Acetyl-L-proline may be used to to identify, differentiate and characterized N-acyl-amino acid amidohydrolase(s)/aminoacylase(s). N-Acetyl-L-proline is used to study the physicochemical parameters of prolines.
N-acetyl-L-proline is an analog of the COOH-terminal dipeptide portion of preferred substrates of angiotensin-converting enzyme (ACE). It may be used in studies of the binding of substrates and inhibitors by ACE and to differentiate the specificities of various aminoacylases.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Abil E Aliev et al.
The journal of physical chemistry. B, 111(50), 14034-14042 (2007-11-22)
The results of the ring conformational analysis of L-proline, N-acetyl-L-proline, and trans-4-hydroxy-L-proline by NMR combined with calculations using density functional theory (DFT) and molecular dynamics (MD) are reported. Accurate values of 1H-1H J-couplings in water and other solvents have been
J Krapcho et al.
Journal of medicinal chemistry, 31(6), 1148-1160 (1988-06-01)
Analogues of captopril, enalaprilat, and the phosphinic acid [hydroxy(4-phenylbutyl)phosphinyl]acetyl]-L-proline incorporating 4-substituted proline derivatives have been synthesized and evaluated as inhibitors of angiotensin-converting enzyme (ACE) in vitro and in vivo. The 4-substituted prolines, incorporating alkyl, aryl, alkoxy, aryloxy, alkylthio, and arylthio
Mayuko Koreishi et al.
Bioscience, biotechnology, and biochemistry, 69(10), 1914-1922 (2005-10-26)
A novel aminoacylase was purified to homogeneity from culture broth of Streptomyces mobaraensis, as evidenced by SDS-polyacrylamide gel electrophoresis (PAGE). The enzyme was a monomer with an approximate molecular mass of 100 kDa. The purified enzyme was inhibited by the
Chiara Cappelli et al.
The journal of physical chemistry. B, 112(11), 3441-3450 (2008-02-26)
The structure and properties of (s)-N-acetylproline amide (NAP) in aqueous solution are studied by exploiting a continuum solvation model. The conformational preference of NAP as a function of the environment is discussed as well as data for a number of
S N Tenjarla et al.
International journal of pharmaceutics, 192(2), 147-158 (1999-11-24)
A series of N-acetylproline esters (alkyl side chain length, 5-18) were synthesized and tested for potential skin penetration enhancement activity using modified Franz diffusion cells and hairless mouse skin as the penetration barrier. Benazepril and hydrocortisone were used as model
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.