17938
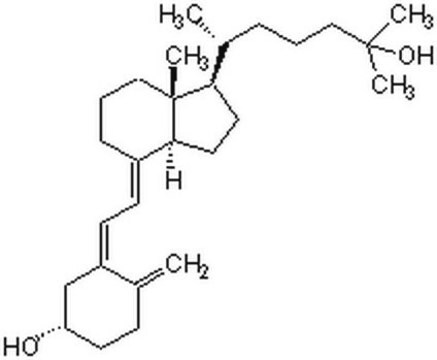
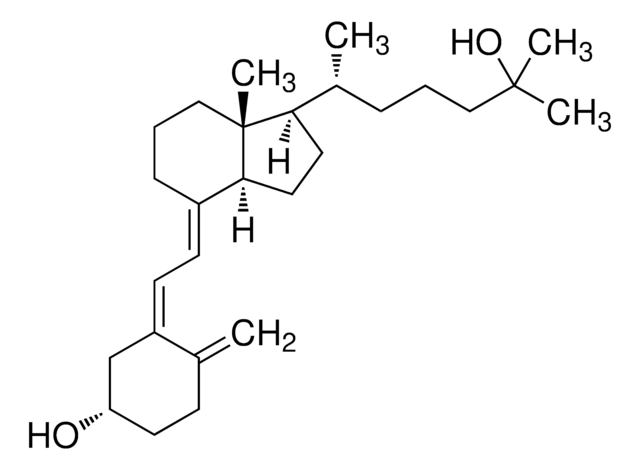
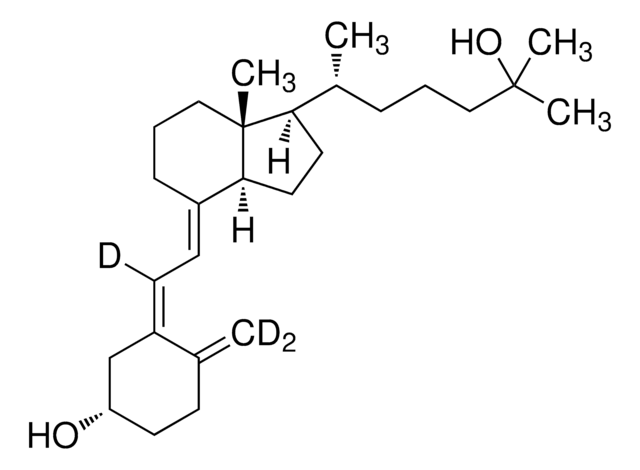
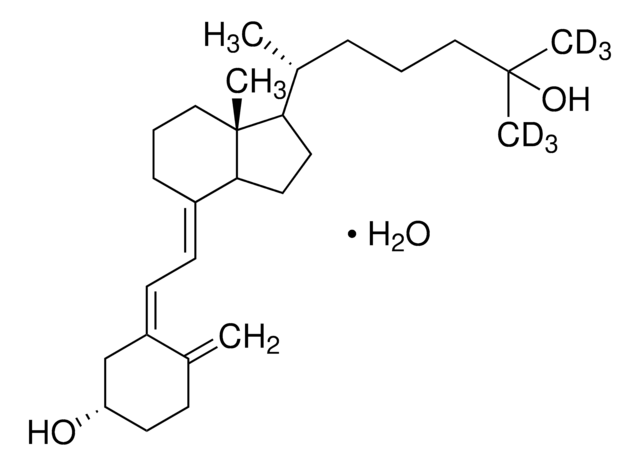
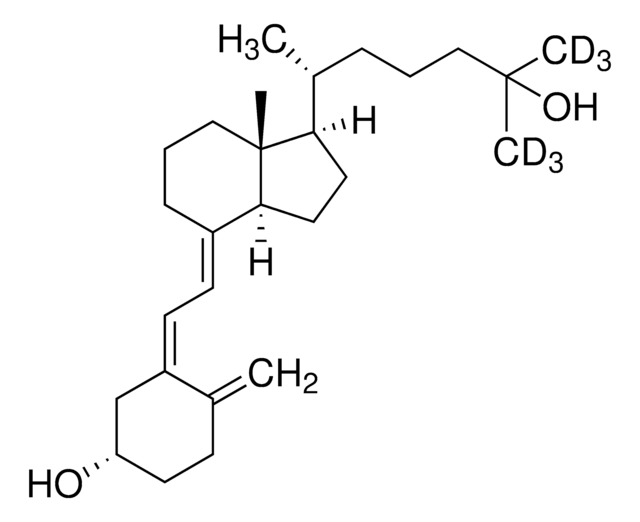
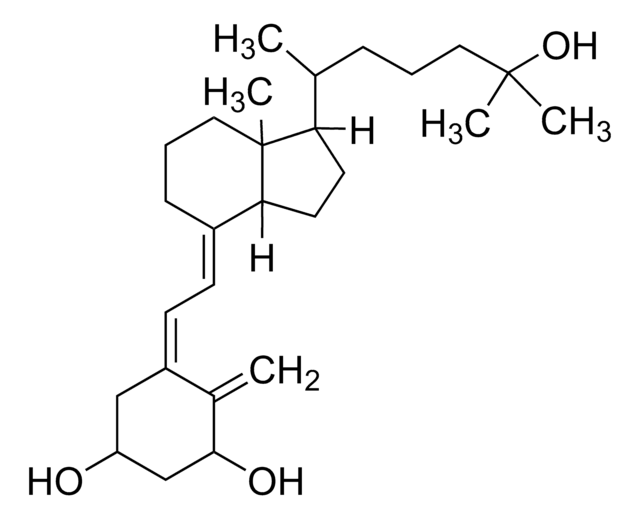
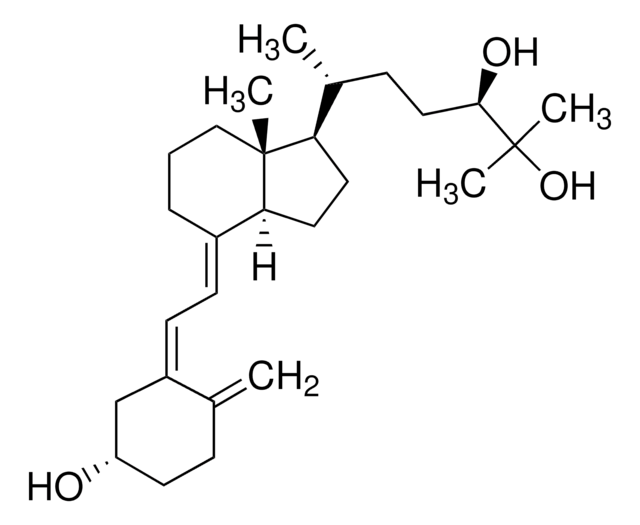
25-Hydroxyvitamin D3 monohydrate
≥99.0% (HPLC)
Sinonimo/i:
25-Hydroxycholecalciferol
About This Item
Prodotti consigliati
Origine biologica
synthetic
Livello qualitativo
Saggio
≥99.0% (HPLC)
Stato
powder or crystals
Colore
white to off-white
Condizioni di spedizione
dry ice
Temperatura di conservazione
−20°C
Stringa SMILE
[H][C@@]1(CC[C@@]2([H])C(\CCC[C@]12C)=C\C=C3\C[C@@H](O)CCC3=C)[C@H](C)CCCC(C)(C)O
InChI
1S/C27H44O2/c1-19-10-13-23(28)18-22(19)12-11-21-9-7-17-27(5)24(14-15-25(21)27)20(2)8-6-16-26(3,4)29/h11-12,20,23-25,28-29H,1,6-10,13-18H2,2-5H3/b21-11+,22-12-/t20-,23+,24-,25+,27-/m1/s1
JWUBBDSIWDLEOM-DTOXIADCSA-N
Informazioni sul gene
human ... VDR(7421)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Azioni biochim/fisiol
Confezionamento
Prodotti correlati
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 2 Inhalation - Acute Tox. 3 Dermal - Acute Tox. 3 Oral - STOT RE 1 Oral
Codice della classe di stoccaggio
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Vitamin D2 (ergocalciferol) is naturally synthesized from ergosterol by invertebrates, fungi, and plants in response to ultraviolet B irradiation, while vitamin D3 synthesis (cholecalciferol) is uniquely initiated in the skin of vertebrates. During sun exposure, ultraviolet B photons are absorbed by 7-dehydrocholesterol, which is found within the plasma membranes of epidermal and dermal skin layers. This reaction yields an unstable derivative of 7-dehydrocholesterol, named precholecalcitrol, which rapidly rearranges to vitamin D3. Vitamin D binding protein (DBP) is a carrier protein responsible for drawing vitamin D3 from the plasma membrane into the dermal capillaries within the extracellular space.
Protocolli
While quantitative analysis was performed for Vitamins D2 and D3, the samples were scanned for the presence of the 25-hydroxy metabolites.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.