186325
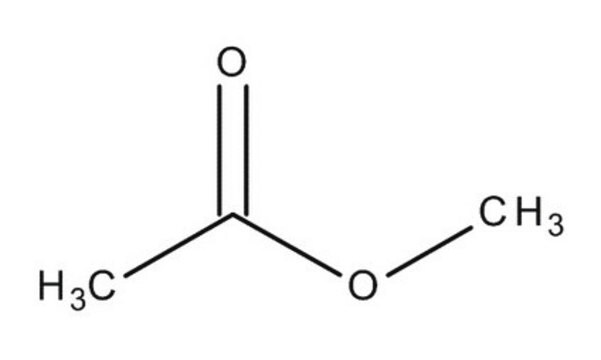
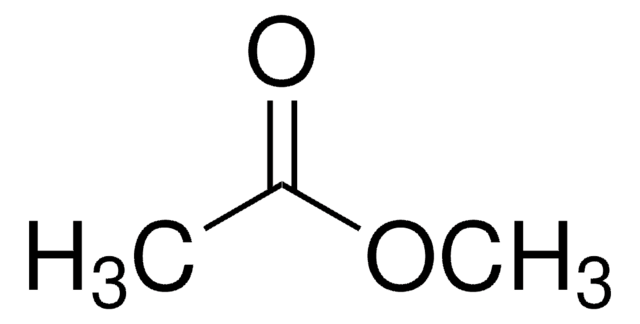
Methyl acetate
ReagentPlus®, 99%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
CH3COOCH3
Numero CAS:
Peso molecolare:
74.08
Beilstein:
1736662
Numero CE:
Numero MDL:
Codice UNSPSC:
12352108
ID PubChem:
NACRES:
NA.21
Prodotti consigliati
Grado
reagent
Livello qualitativo
Densità del vapore
2.55 (vs air)
Tensione di vapore
165 mmHg ( 20 °C)
Nome Commerciale
ReagentPlus®
Saggio
99%
Stato
liquid
Temp. autoaccensione
936 °F
Limite di esplosione
16 %
dilution
(for general lab use)
Indice di rifrazione
n20/D 1.361 (lit.)
P. ebollizione
57-58 °C (lit.)
Punto di fusione
−98 °C (lit.)
Densità
0.934 g/mL at 25 °C
Stringa SMILE
COC(C)=O
InChI
1S/C3H6O2/c1-3(4)5-2/h1-2H3
KXKVLQRXCPHEJC-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Its IR spectra in the vapor phase and in solution form (in CS2 and CCl4) have been reported. It can be synthesized from dimethyl ether via carbonylation in the presence of halide-free catalysts based on zeolites. It has also been reported to be formed during the synthesis of poly(vinyl) alcohol (PVA). It undergoes transesterification reaction with n-octanol in the presence of Amberlyst 15 catalyst to afford octyl acetate and methanol.
Methyl acetate is an aliphatic ester that can be prepared via carbonylation of dimethyl ether over zeolites. MA is formed as a by-product during the preparation of polyvinyl alcohol from acetic acid and methanol.
Applicazioni
Methyl acetate may be used for the preparation of fatty acid methyl esters and triacetin from rapeseed oil via non-catalytic trans-esterification reaction under super-critical conditions.
Methyl acetate may be used in the following:
- As acyl acceptor in the preparation of biodiesel.
- Synthesis of ethanol.
- Preparation of n-butyl acetate, via transesterification reaction with n-butanol in the presence of acidic catalysts.
- acetic anhydride
- methyl acrylate
- vinyl acetate
- ethyl amide
Note legali
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
Organi bersaglio
Central nervous system
Rischi supp
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
8.6 °F - closed cup
Punto d’infiammabilità (°C)
-13 °C - closed cup
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Kinetics of transesterification of methyl acetate and n-octanol catalyzed by cation exchange resins.
Liu Y, et al.
Korean Journal of Chemical Engineering, 30(5), 1039-1042 (2013)
Catalysts, Kinetics, and Reactive Distillation for Methyl Acetate Synthesis.
Zuo C, et al.
Industrial & Engineering Chemistry Research, 53(26), 10540-10548 (2014)
A new process for catalyst-free production of biodiesel using supercritical methyl acetate.
Saka S and Isayama Y.
Fuel: The Science and Technology of Fuel and Energy, 88(7), 1307-1313 (2009)
Selective carbonylation of dimethyl ether to methyl acetate catalyzed by acidic zeolites.
Patricia Cheung et al.
Angewandte Chemie (International ed. in English), 45(10), 1617-1620 (2006-01-31)
Synthesis of ethanol from methanol and syngas through an indirect route containing methanol dehydrogenation, DME carbonylation, and methyl acetate hydrogenolysis.
Liu Y, et al.
Fuel Processing Technology, 110, 206-213 (2013)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.