159417
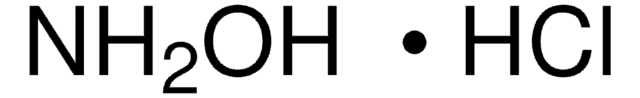
Hydroxylamine hydrochloride
ReagentPlus®, 99%
Sinonimo/i:
Hydroxylammonium chloride
About This Item
Prodotti consigliati
Tensione di vapore
0.001 hPa ( 50 °C)
Livello qualitativo
Nome Commerciale
ReagentPlus®
Saggio
99%
Forma fisica
crystalline
tecniche
inhibition assay: suitable
pH
2.5-3.5 (20 °C, 50 g/L)
Punto di fusione
155-157 °C (dec.) (lit.)
Densità
1.67 g/mL at 25 °C (lit.)
Stringa SMILE
Cl.NO
InChI
1S/ClH.H3NO/c;1-2/h1H;2H,1H2
WTDHULULXKLSOZ-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- in the synthesis of primary amides from aldehydes in the presence of cesium carbonate (Cs2CO3) as a catalyst.
- in the conversion of alicyclic /aliphatic carbonyl compounds and the aromatic aldehydes into corresponding oximes.
- in the one-pot synthesis of nitriles from aldehydes in the presence of sodium sulfate (anhyd) and sodium bicarbonate catalysts.
- It can also be used as a reducing agent in the preparation of single-layer reduced graphene oxide (RGO) sheets and films.
Azioni biochim/fisiol
Note legali
Avvertenze
Warning
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Carc. 2 - Eye Irrit. 2 - Met. Corr. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 2 Oral
Organi bersaglio
spleen
Codice della classe di stoccaggio
4.1A - Other explosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.