SMB01357
Curcumin glucuronide
Sinonimo/i:
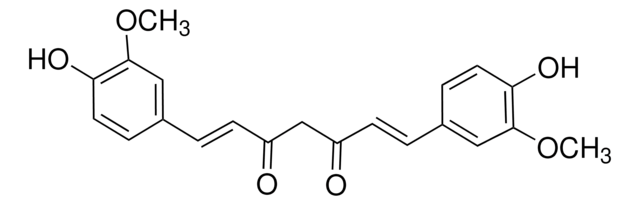
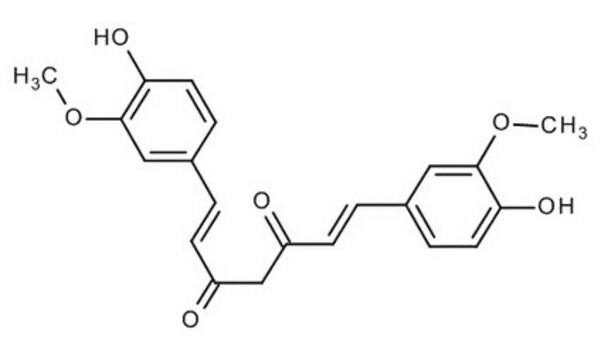
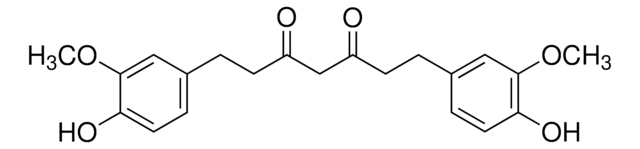
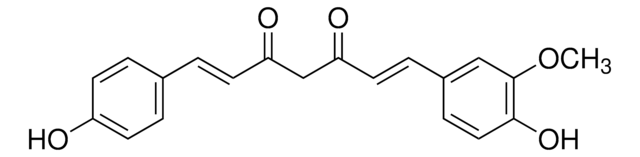
4-[(1E,6E)-7-(4-Hydroxy-3-methoxyphenyl)-3,5-dioxo-1,6-heptadien-1-yl]-2-methoxyphenyl β-D-glucopyranosiduronic acid, Curcumin-beta-D-glucuronide
About This Item
Prodotti consigliati
Forma fisica
powder
Livello qualitativo
Temperatura di conservazione
2-8°C
Stringa SMILE
O[C@@H]1[C@@H](O)[C@H](OC2=CC=C(/C=C/C(CC(/C=C/C3=CC=C(O)C(OC)=C3)=O)=O)C=C2OC)O[C@H](C(O)=O)[C@H]1O
InChI
1S/C27H28O12/c1-36-20-11-14(5-9-18(20)30)3-7-16(28)13-17(29)8-4-15-6-10-19(21(12-15)37-2)38-27-24(33)22(31)23(32)25(39-27)26(34)35/h3-12,22-25,27,30-33H,13H2,1-2H3,(H,34,35)/b7-3+,8-4+/t22-,23-,24+,25-,27+/m0/s1
BNSAVBGHRVFVNN-XSCLDSQRSA-N
Descrizione generale
Applicazioni
Azioni biochim/fisiol
Caratteristiche e vantaggi
- High quality compound suitable for multiple research applications
- Compatible with a wide variety of chromatographic and spectrometry techniques
Altre note
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Ci dispiace, ma al momento non ci sono COA disponibili online per questo prodotto.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.