PHR1876
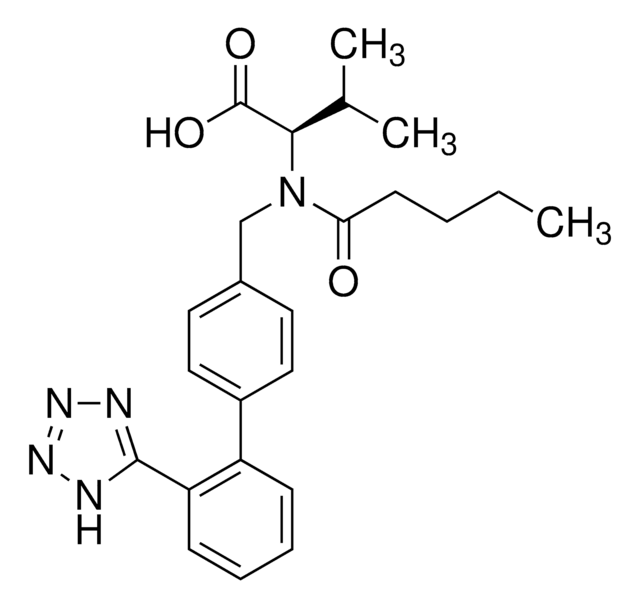
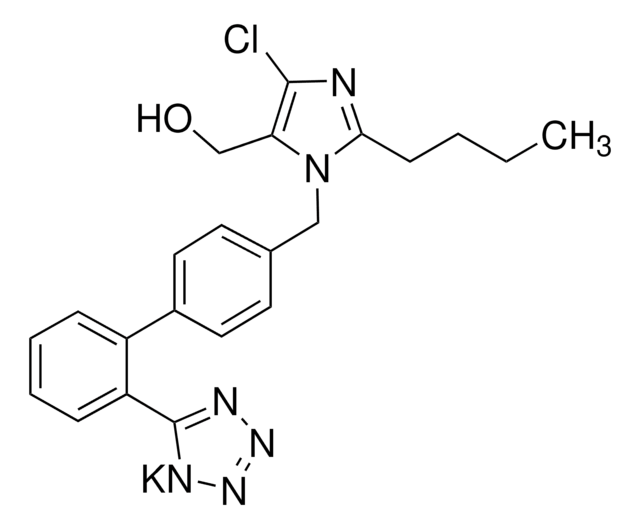
Valsartan Related Compound B
Pharmaceutical Secondary Standard; Certified Reference Material
Sinonimo/i:
N-(1-Oxobutyl)-N-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-L-valine, N-butyryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]methyl}-L-valine, (S)-N-Butyryl-N-{[2′-(1-H-tetrazole-5-yl)-biphenyl-4-yl]methyl}valine
About This Item
Prodotti consigliati
Grado
certified reference material
pharmaceutical secondary standard
Livello qualitativo
agenzia
traceable to USP 1708784
Famiglia di API
valsartan
CdA
current certificate can be downloaded
Confezionamento
pkg of 30 mg
applicazioni
pharmaceutical
Formato
neat
Temperatura di conservazione
2-8°C
Stringa SMILE
CCCC(=O)N(Cc1ccc(cc1)-c2ccccc2-c3nnn[nH]3)[C@@H](C(C)C)C(O)=O
InChI
1S/C23H27N5O3/c1-4-7-20(29)28(21(15(2)3)23(30)31)14-16-10-12-17(13-11-16)18-8-5-6-9-19(18)22-24-26-27-25-22/h5-6,8-13,15,21H,4,7,14H2,1-3H3,(H,30,31)(H,24,25,26,27)/t21-/m0/s1
OKAQHVJSXLGXET-NRFANRHFSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
It is an impurity of the potent, highly selective, and orally active antagonist of the angiotensin II AT1-receptor, valsartan, used widely for the treatment of hypertension.
Applicazioni
- Development of a reversed-phase high-performance liquid chromatographic (RP-HPLC) method for the determination of valsartan and its related impurities in pharmaceutical dosage forms
- Impurity testing of valsartan, amlodipine besylate, and hydrochlorothiazide in their combined dosage form by a stability-indicating ultra-high performance liquid chromatography (UHPLC)
- Simultaneous determination of amlodipine and valsartan in their combined dosage form, in the presence of their degradation products by a gradient reversed phase-liquid chromatographic (RP-LC) method
- Separation and detection of nitrosamines and other related impurities in valsartan and losartan using supercritical fluid chromatography (SFC) in a single run
- Development and validation of a UHPLC method for the estimation of sacubitril, valsartan, and their related impurities in their combined dosage form, following ICH Q2 (R1) guideline
Risultati analitici
Nota a piè di pagina
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Repr. 2 - STOT SE 3
Organi bersaglio
Central nervous system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documenti section.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.