1708784
USP
Valsartan Related Compound B
United States Pharmacopeia (USP) Reference Standard
Sinonimo/i:
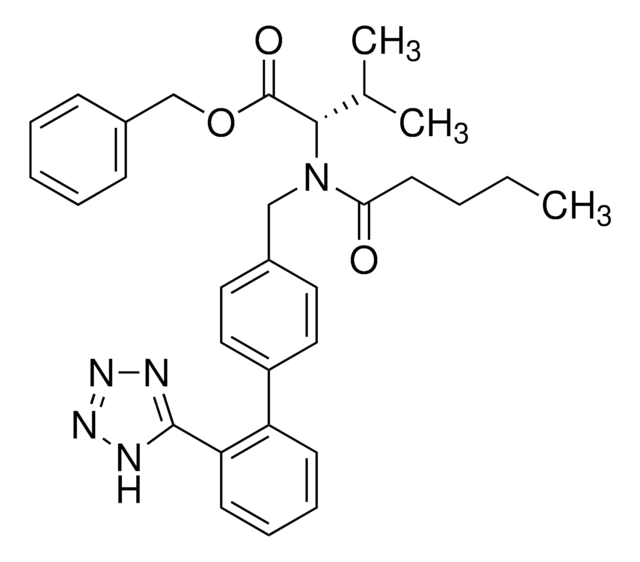
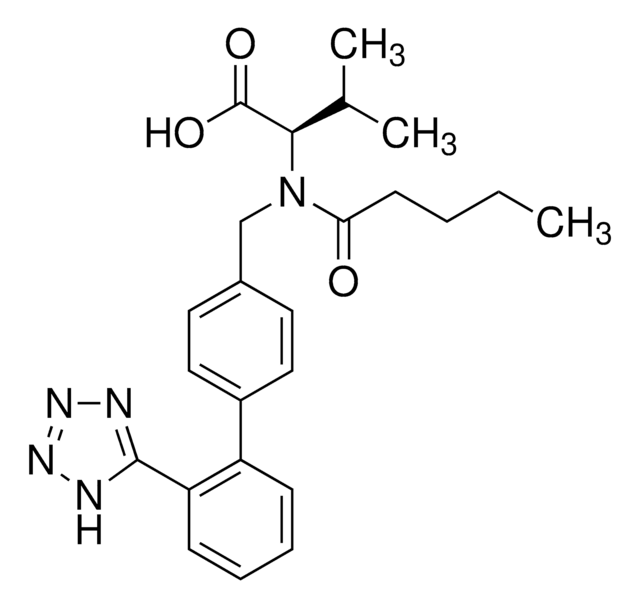
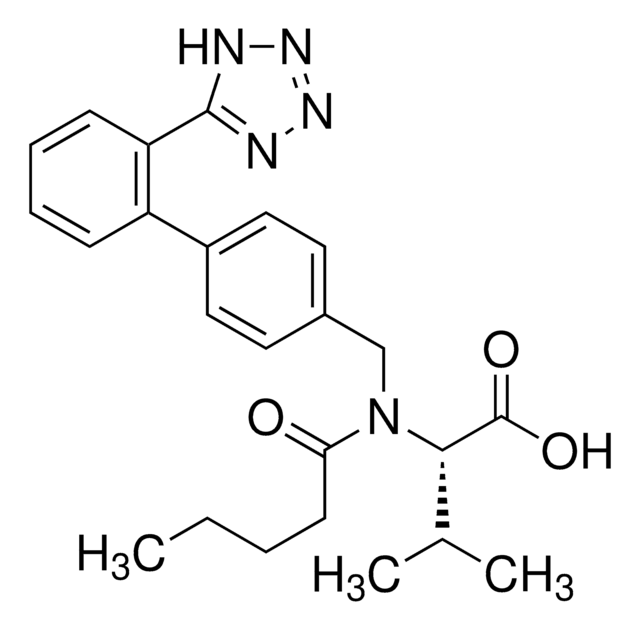
N-(1-Oxobutyl)-N-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-L-valine, (S)-N-Butyryl-N-{[2′-(1-H-tetrazole-5-yl)-biphenyl-4-yl]methyl}valine
About This Item
Prodotti consigliati
Grado
pharmaceutical primary standard
Famiglia di API
valsartan
Produttore/marchio commerciale
USP
applicazioni
pharmaceutical (small molecule)
Formato
neat
Stringa SMILE
CCCC(=O)N(Cc1ccc(cc1)-c2ccccc2-c3nnn[nH]3)[C@@H](C(C)C)C(O)=O
InChI
1S/C23H27N5O3/c1-4-7-20(29)28(21(15(2)3)23(30)31)14-16-10-12-17(13-11-16)18-8-5-6-9-19(18)22-24-26-27-25-22/h5-6,8-13,15,21H,4,7,14H2,1-3H3,(H,30,31)(H,24,25,26,27)/t21-/m0/s1
OKAQHVJSXLGXET-NRFANRHFSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
It is also used in the preparation of standard and system suitability solutions for the determination of organic impurities and performance tests of tablets and capsules, in accordance with the USP monographs, by liquid chromatography (LC) combined with UV detection, of the following:
- Valsartan
- Amlodipine and valsartan tablets
- Valsartan and hydrochlorothiazide tablets
- Amlodipine, valsartan, and hydrochlorothiazide tablets
In addition, it is used in the assay test of valsartan tablets by LC-UV following the USP monograph “Valsartan Tablets”.
Risultati analitici
Altre note
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Repr. 2 - STOT SE 3
Organi bersaglio
Central nervous system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.