71210
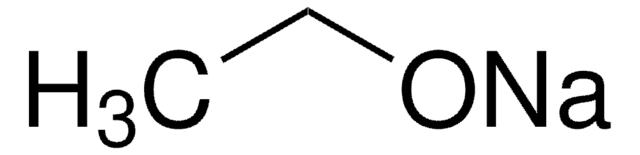
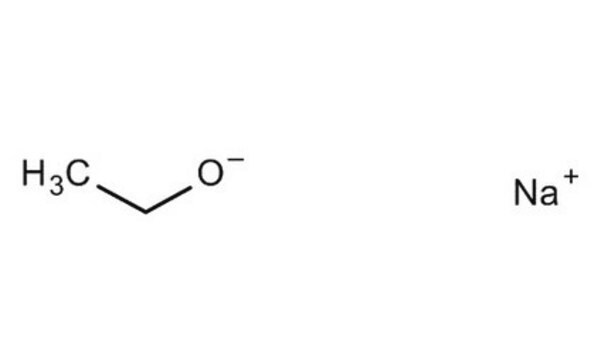
Sodium ethoxide
technical, ≥95% (T)
Sinonimo/i:
Sodium ethylate
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
CH3CH2ONa
Numero CAS:
Peso molecolare:
68.05
Beilstein:
3593646
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.21
Prodotti consigliati
Densità del vapore
1.6 (vs air)
Livello qualitativo
Tensione di vapore
<0.1 mmHg ( 20 °C)
Grado
technical
Saggio
≥95% (T)
Forma fisica
powder
Impurezze
~2% Na2CO3 and NaOH
Stringa SMILE
[Na+].CC[O-]
InChI
1S/C2H5O.Na/c1-2-3;/h2H2,1H3;/q-1;+1
QDRKDTQENPPHOJ-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Sodium ethoxide (Sodium ethylate) is a sodium alkoxide. It has been synthesized by reacting sodium with ethanol. It undergoes decomposition in the presence of water to afford ethanol and sodium hydroxide. It is widely employed as a strong base in organic synthesis studies.
Sodium ethoxide is an alkoxide salt mainly used as a strong base in organic reactions such as deprotonation, dehydration and dehalogenation.
Applicazioni
Sodium ethoxide may be used as a base for the palladium catalyzed cross-coupling of aryl halides and alkenylboranes to synthesize arylated (E)-alkenes.
Sodium ethoxide may be used for the preparation of tricarbonylchloro(glycinato)ruthenium(II) (CORM-3).
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Flam. Sol. 1 - Self-heat. 1 - Skin Corr. 1A
Rischi supp
Codice della classe di stoccaggio
4.2 - Pyrophoric and self-heating hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
86.0 °F - closed cup
Punto d’infiammabilità (°C)
30 °C - closed cup
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Whitaker KS and Whitaker DT
e-EROS Encyclopedia of Reagents for Organic Synthesis (2001)
Stereoselective synthesis of arylated (E)-alkenes by the reaction of alk-1-enylboranes with aryl halides in the presence of palladium catalyst.
Miyaura N & Suzuki, A.
Journal of the Chemical Society. Chemical Communications, 19, 866-867 (1979)
James E Clark et al.
Circulation research, 93(2), e2-e8 (2003-07-05)
Carbon monoxide, which is generated in mammals during the degradation of heme by the enzyme heme oxygenase, is an important signaling mediator. Transition metal carbonyls have been recently shown to function as carbon monoxide-releasing molecules (CO-RMs) and to elicit distinct
Eagleson M.
Concise Encyclopedia Chemistry, 997-997 (1994)
Ali Reza Harifi-Mood et al.
Journal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques, 7(1), 88-91 (2004-05-18)
Variable-Temperature Kinetics has been used to obtain the rate constants of the reaction at various temperatures during one kinetic run. Pseudo-first-order rate constants for the transesterification of procaine with aliphatic alcohols ethanol, n-propanol and tert-butanol were obtained by the fluorescence
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.