30552
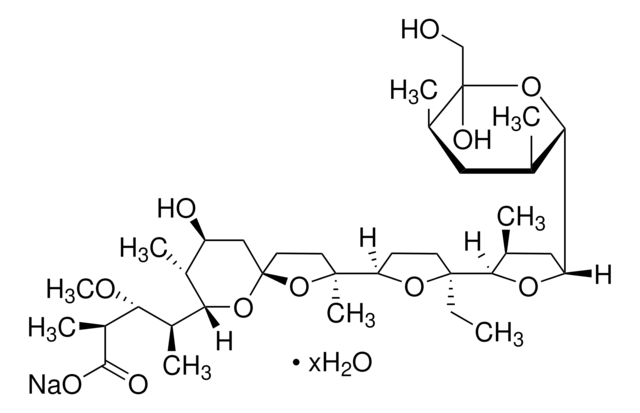
Monensin methyl ester
Selectophore™, ≥97.0% (TLC)
Sinonimo/i:
Methyl monensin
About This Item
Prodotti consigliati
Grado
for ion-selective electrodes
Livello qualitativo
Nome Commerciale
Selectophore™
Saggio
≥97.0% (TLC)
Temperatura di conservazione
2-8°C
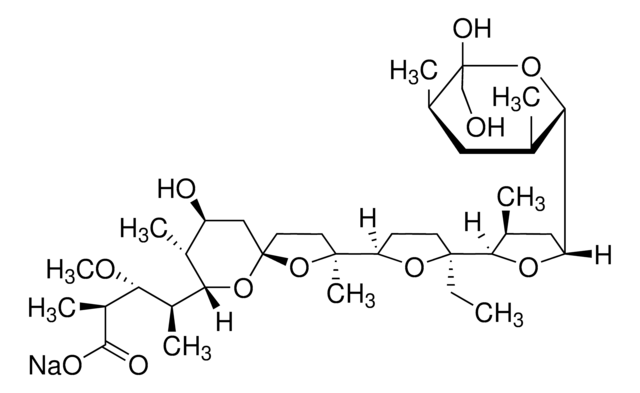
Stringa SMILE
CC[C@]1(CCC(O1)[C@]2(C)CC[C@]3(C[C@H](O)[C@@H](C)C(O3)[C@@H](C)[C@@H](OC)[C@H](C)C(=O)OC)O2)C4OC(C[C@@H]4C)C5O[C@@](O)(CO)[C@H](C)C[C@@H]5C
InChI
1S/C37H64O11/c1-11-35(32-21(3)17-27(44-32)29-20(2)16-22(4)37(41,19-38)47-29)13-12-28(45-35)34(8)14-15-36(48-34)18-26(39)23(5)31(46-36)24(6)30(42-9)25(7)33(40)43-10/h20-32,38-39,41H,11-19H2,1-10H3/t20-,21+,22+,23+,24+,25+,26-,27-,28+,29-,30-,31-,32-,34+,35-,36+,37-/m0/s1
PFRZSHIENRKVSE-RJTHVKINSA-N
Categorie correlate
Descrizione generale
Applicazioni
- The selectivity of membrane ion-selective electrodes: This research explores the use of temperature variations to adjust the selectivity of ion-selective electrodes, utilizing Monensin methyl ester as a key component for sodium ion detection (Zahran et al., 2010).
- Spectroscopic and semiempirical studies of a proton channel formed by the methyl ester of monensin A.: This paper presents a detailed analysis of the proton channel properties of Monensin methyl ester through spectroscopic and computational methods, highlighting its potential in analytical chemistry applications (Huczyński et al., 2006).
- Ion chromatography detector based on solid-state ion-selective electrode array.: The development of an ion chromatography detector employing solid-state ion-selective electrodes, with Monensin methyl ester playing a crucial role in sodium ion detection, is detailed in this study (Lee et al., 2000).
Confezionamento
Note legali
Prodotti correlati
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Oral
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documenti section.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.