342001
Procathepsin K, Human, Recombinant, E. coli
Recombinant, human procathepsin K expressed in E. coli with a methionine residue inserted at amino acid 18 to create a new N-terminal initiation site.
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Prodotti consigliati
Ricombinante
expressed in E. coli
Livello qualitativo
Saggio
≥95% (SDS-PAGE)
Stato
liquid
Attività specifica
≥1000 mU/mg protein
Produttore/marchio commerciale
Calbiochem®
Condizioni di stoccaggio
OK to freeze
avoid repeated freeze/thaw cycles
Condizioni di spedizione
wet ice
Temperatura di conservazione
−70°C
Descrizione generale
If the activated enzyme is not used immediately, it is recommended to add methyl methanthiosulfonate (1 mM final concentration MMTS).
Note: 1 mU = 1 milliunit.
Recombinant, human procathepsin K (amino acids 19-329) (GenBank target symbol = S79895, ACC No. P43235) expressed in E. coli with a methionine residue inserted at amino acid 18 to create a new N-terminal initiation site. Cathepsin K, a member of the papain superfamily of cysteine proteinases, plays an important role in osteoclast-mediated bone resorption and collagen degradation. Cathepsin K is synthesized as an inactive proenzyme that is converted to its mature, active form by proteolytic cleavage of the 99 amino acid propeptide domain. Inhibitors of cathepsin K include leupeptin (Cat. No. 108975) (IC50 = 70 nM), E-64 (Cat. No. 324890) (IC50 = 5 nM), and cystatin (Cat. No. 324891 or 324896). Requires activation prior to use.
Confezionamento
Please refer to vial label for lot-specific concentration.
Attenzione
Toxicity: Standard Handling (A)
Definizione di unità
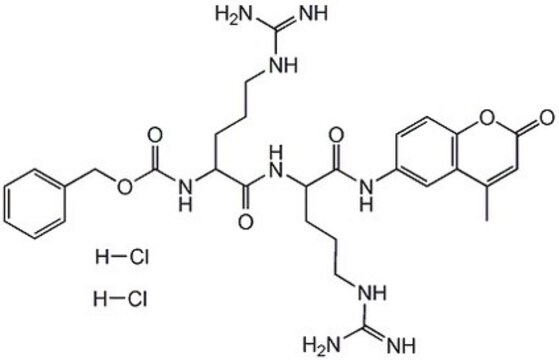
One unit is defined as the amount of enzyme that will hydrolyze 1 µmole benzyloxycarbonyl-phenylalanine-arginine-7-amido-4-methylcoumarin per min at 37°C, pH 5.5.
Stato fisico
In 500 mM NaCl, 25 mM Tris, pH 8.0.
Ricostituzione
Following initial thaw, aliquot and freeze (-70°C). Following activation the enzyme is unstable and should include MMTS for storage (see recommended reaction conditions for activation).
Altre note
McQueney, M., et al. 1997. J. Bio. Chem.272, 13955.
Bossard, M., et al. 1996. J. Biol. Chem.271, 12517.
Drake, F., et al. 1996. J. Biol. Chem.271, 12511.
Bromme, D. and Okamoto, K. 1995. Biol. Chem. Hoppe-Seyler376, 379.
Baron, R. 1989 Anat. Rec.224, 317.
Littlewood-Evans, A.J., et al. 1975. Cancer Res.57, 5386.
Nishimura, J.S. et al. 1975. Arch. Biochem. Biophys.170, 461.
Bossard, M., et al. 1996. J. Biol. Chem.271, 12517.
Drake, F., et al. 1996. J. Biol. Chem.271, 12511.
Bromme, D. and Okamoto, K. 1995. Biol. Chem. Hoppe-Seyler376, 379.
Baron, R. 1989 Anat. Rec.224, 317.
Littlewood-Evans, A.J., et al. 1975. Cancer Res.57, 5386.
Nishimura, J.S. et al. 1975. Arch. Biochem. Biophys.170, 461.
Note legali
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Codice della classe di stoccaggio
12 - Non Combustible Liquids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.