X600
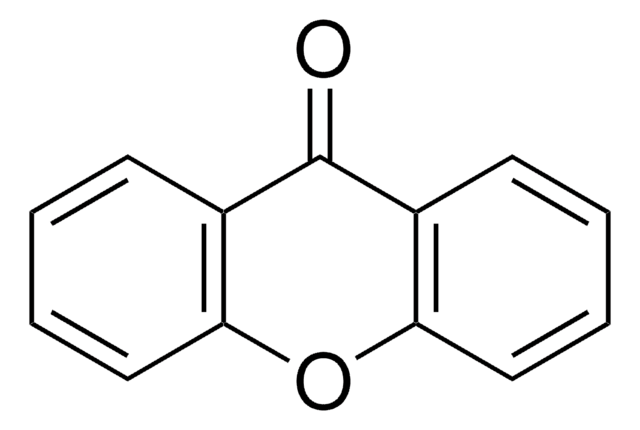
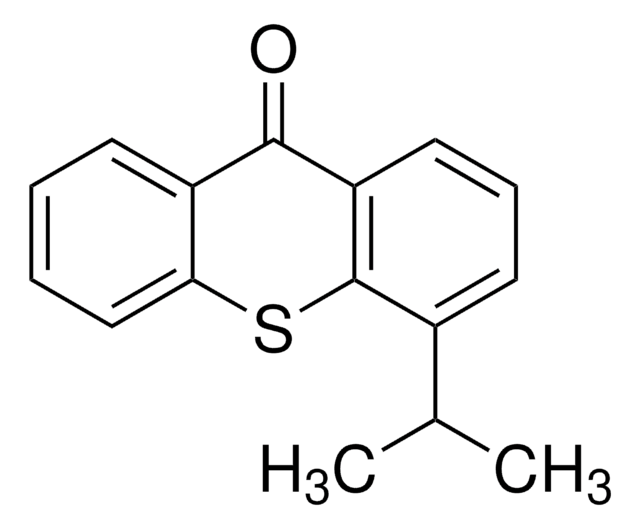
Xanthone
97%
Sinonimo/i:
9-Xanthenone
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C13H8O2
Numero CAS:
Peso molecolare:
196.20
Beilstein:
140443
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
Stato
powder
P. ebollizione
349-350 °C/730 mmHg (lit.)
Punto di fusione
172-174 °C (lit.)
Stringa SMILE
O=C1c2ccccc2Oc3ccccc13
InChI
1S/C13H8O2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1-8H
JNELGWHKGNBSMD-UHFFFAOYSA-N
Informazioni sul gene
mouse ... Prkch(18755)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Rajan Giri et al.
Bioorganic & medicinal chemistry, 18(4), 1456-1463 (2010-02-05)
A series of substituted xanthenes was synthesized and screened for activity using DU-145, MCF-7, and HeLa cancer cell growth inhibition assays. The most potent compound, 9 g ([N,N-diethyl]-9-hydroxy-9-(3-methoxyphenyl)-9H-xanthene-3-carboxamide), was found to inhibit cancer cell growth with IC(50) values ranging from
Asako Murata et al.
Bioorganic & medicinal chemistry letters, 23(1), 252-255 (2012-11-21)
In recent years, various biological processes have been found to be regulated by miRNA-mediated gene silencing. A small molecule that modulate the miRNA pathway will provide the biological tool for elucidating mechanisms of miRNA-mediated gene regulation, and can be the
Krishna Kanta Ghosh et al.
Chemical communications (Cambridge, England), 47(26), 7488-7490 (2011-06-02)
We report the first solid phase synthesis of a xanthone library CX and its application to embryonic stem cell probe development. The CX library was further derivatised with an activated ester resin to provide an acetylated CX (CXAC) library. Screening
Michael A Schätzle et al.
Journal of the American Chemical Society, 134(36), 14742-14745 (2012-08-23)
Reduction of emodin by sodium dithionite resulted in the formation of two tautomeric forms of emodin hydroquinone. Subsequent conversion by the short-chain dehydrogenase/reductase (SDR) MdpC into the corresponding 3-hydroxy-3,4-dihydroanthracen-1(2H)-one implies that deoxygenation is the first step in monodictyphenone biosynthesis. Implications
Jessie A Blake et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 11(3), 539-547 (2012-01-10)
We have attached the antiviral drug acyclovir (ACV) to a xanthone photolabile protecting group (or photocage) through the O6 position of acyclovir, a procedure designed for the treatment of ocular herpes simplex virus infections. Acyclovir is photoreleased from the photocage
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.