D80002
DCC
99%
Sinonimo/i:
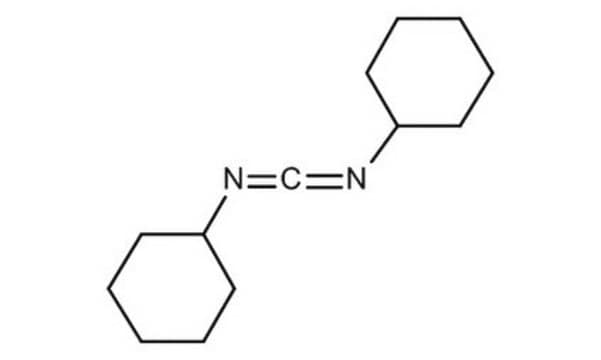
N,N′-Dicyclohexylcarbodiimide
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
99%
Forma fisica
solid
Impiego in reazioni chimiche
reaction type: Coupling Reactions
P. eboll.
122-124 °C/6 mmHg (lit.)
Punto di fusione
34-35 °C (lit.)
Stringa SMILE
C1CCC(CC1)N=C=NC2CCCCC2
InChI
1S/C13H22N2/c1-3-7-12(8-4-1)14-11-15-13-9-5-2-6-10-13/h12-13H,1-10H2
QOSSAOTZNIDXMA-UHFFFAOYSA-N
Informazioni sul gene
human ... EPHX2(2053)
mouse ... Ephx2(13850)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
7-((tert-butyldimethylsilyl)oxy)hepta-2,4-diyn-1-ol with propiolic acid to form 7-((tert-butyldimethylsilyl)oxy)hepta-2,4-diyn-1-yl propynoate.
It can also used to synthesize:
- 1,3-Thiazetedine derivatives via [2+2] cycloaddition with 2-phenylethenyl- and 2-(4-nitrophenyl)ethenyl isothiocyanates.
- 1,3,5-Oxadiazine-4-thiones via [4+2] cycloaddition with benzoyl isothiocyanates.
- Sterically hindered 1,3,4-oxadiazole derivatives by reacting with (N-isocyanimino)triphenylphosphorane in the presence of aromatic (or heteroaromatic) carboxylic acids.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Sens. 1
Codice della classe di stoccaggio
6.1D - Non-combustible, acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
235.4 °F - closed cup
Punto d’infiammabilità (°C)
113 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Carbodiimide-mediated peptide coupling remains to the most frequently used technique.
Professor Aran (Claremont University, USA) thoroughly discusses the engineering of graphene based materials through careful functionalization of graphene oxide, a solution processable form of graphene.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.