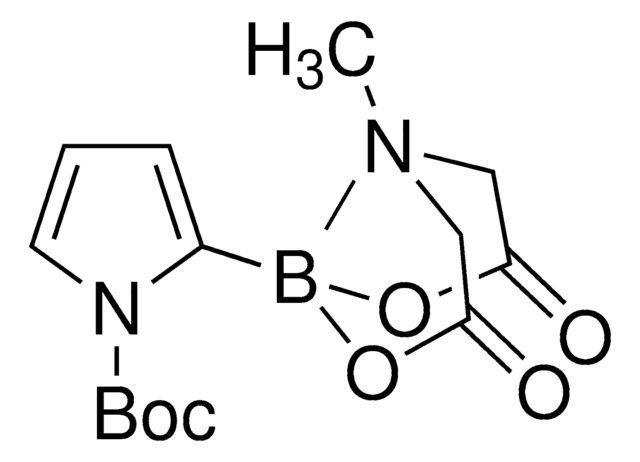
930466
HMP-alkyne
≥95%
Sinonimo/i:
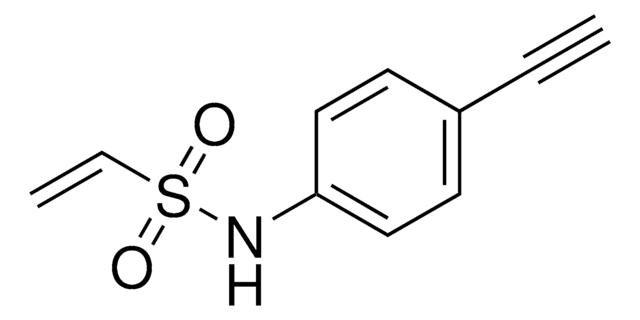
4-Hydroxy-3-(hydroxymethyl)-N-(prop-2-yn-1-yl)benzenesulfonamide
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C10H11NO4S
Peso molecolare:
241.26
Codice UNSPSC:
12352101
NACRES:
NA.22
Prodotti consigliati
Descrizione
Application: Chemoproteomics
Livello qualitativo
Saggio
≥95%
Forma fisica
powder or chunks
Temperatura di conservazione
−20°C
Stringa SMILE
OCC1=C(O)C=CC(S(NCC#C)(=O)=O)=C1
Applicazioni
HMP-alkyne is a probe that can be used to photochemically label tryptophans. A method was developed using cysteine-reactive compounds including this one to allow for unbiased analysis of proteomic data in quantitative applications (Zanon et al. 2021). The method uses light or heavy labeling with the isotopically labelled desthiobiotin azide (isoDTB) tag for mass spectrometry analysis (Zanon et al. 2020). Analysis then uses the isotopic tandem orthogonal proteolysis activity-based protein profiling (isoTOP-ABPP) workflow (Weerapana et al. 2010, Backus et al. 2016)
Altre note
Profiling the proteome-wide selectivity of diverse electrophiles
A quantitative thiol reactivity profiling platform to analyze redox and electrophile reactive cysteine proteomes
Ethynylation of Cysteine Residues: From Peptides to Proteins in Vitro and in Living Cells
A Chemoproteomic Platform To Assess Bioactivation Potential of Drugs
Inhibition of Zinc-Dependent Histone Deacetylases with a Chemically Triggered Electrophile
Reversibility of Covalent Electrophile-Protein Adducts and Chemical Toxicity
A quantitative thiol reactivity profiling platform to analyze redox and electrophile reactive cysteine proteomes
Ethynylation of Cysteine Residues: From Peptides to Proteins in Vitro and in Living Cells
A Chemoproteomic Platform To Assess Bioactivation Potential of Drugs
Inhibition of Zinc-Dependent Histone Deacetylases with a Chemically Triggered Electrophile
Reversibility of Covalent Electrophile-Protein Adducts and Chemical Toxicity
Prodotti correlati
N° Catalogo
Descrizione
Determinazione del prezzo
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Lot/Batch Number
Ci dispiace, ma al momento non ci sono COA disponibili online per questo prodotto.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Ping Yu et al.
Nucleosides, nucleotides & nucleic acids, 40(7), 754-766 (2021-06-29)
We report herein comprehensive investigations of alkylation/sulfur exchange reactions of sulfur-containing substrates including nucleosides such as s2U, m5s2U, s4U, s2A and s2T-incorporated DNA enable by comprehensive screenings of the reagents (2a-2h). It has been proven that iodoacetamide (2a) displays the
Rui Sun et al.
Chemical research in toxicology, 30(10), 1797-1803 (2017-09-30)
Reactive metabolites (RM) formed from bioactivation of drugs can covalently modify liver proteins and cause mechanism-based inactivation of major cytochrome P450 (CYP450) enzymes. Risk of bioactivation of a test compound is routinely examined as part of lead optimization efforts in
Yide He et al.
Talanta, 134, 468-475 (2015-01-27)
In this work, we present a two-step labeling approach for the efficient tagging with lanthanide-containing complexes. For this purpose, derivatization of the cysteine residues with an alkyne group acting as linker was done before the DOTA complex was introduced using
Zarko V Boskovic et al.
ACS chemical biology, 11(7), 1844-1851 (2016-04-12)
Unbiased binding assays involving small-molecule microarrays were used to identify compounds that display unique patterns of selectivity among members of the zinc-dependent histone deacetylase family of enzymes. A novel, hydroxyquinoline-containing compound, BRD4354, was shown to preferentially inhibit activity of HDAC5
Yide He et al.
Journal of proteomics, 136, 68-76 (2015-12-30)
In a proof of concept study, metal-coded affinity tags based on click chemistry (MeCAT-Click) were used to analyze the proteome of Escherichia coli (E. coli) in response to heat stress. This allows high labeling efficiency, high detection sensitivity, and multiplex
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.
![Poly[(9,9-dioctylfluorenyl-2,7-diyl)-alt-(6,6′-{2,2′-bipyridine})] Mw ≥10,000 Da by GPC](/deepweb/assets/sigmaaldrich/product/structures/229/416/b7bc5f74-105e-4593-b0f8-f605aee79aec/640/b7bc5f74-105e-4593-b0f8-f605aee79aec.png)