905313
Rh2(S-TCPTAD)4
Sinonimo/i:
Davies dirhodium catalyst
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C80H64Cl16N4O16Rh2
Numero CAS:
Peso molecolare:
2110.44
Codice UNSPSC:
12352200
Prodotti consigliati
Forma fisica
powder or crystals
Categorie correlate
Applicazioni
This dirhodium catalyst developed by the Davies lab can form C-C bonds at the most accessible tertiary C-H position with control of both regioselectivity and absolute configuration.
Altre note
Formation of Tertiary Alcohols from the Rhodium-Catalyzed Reactions of Donor/Acceptor Carbenes with Esters
Harnessing the β-Silicon Effect for Regioselective and Stereoselective Rhodium(II)-Catalyzed C-H Functionalization by Donor/Acceptor Carbenes Derived from 1-Sulfonyl-1,2,3-triazoles
Site-selective and stereoselective functionalization of non-activated tertiary C-H bonds
Harnessing the β-Silicon Effect for Regioselective and Stereoselective Rhodium(II)-Catalyzed C-H Functionalization by Donor/Acceptor Carbenes Derived from 1-Sulfonyl-1,2,3-triazoles
Site-selective and stereoselective functionalization of non-activated tertiary C-H bonds
Prodotti correlati
N° Catalogo
Descrizione
Determinazione del prezzo
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Enantioselective intramolecular aza-spiroannulation onto benzofurans using chiral rhodium catalysis.
Shibuta T, et al.
Heterocycles, 89, 631-639 (2014)
Liangbing Fu et al.
Organic letters, 16(11), 3036-3039 (2014-05-21)
A rhodium-catalyzed asymmetric synthesis of β-lactones via intramolecular C-H insertion into the ester group of aryldiazoacetates has been developed. The β-lactones were synthesized in high yields and with high levels of diastereo- and enantioselectivity. Halo and trifluoromethyl substituents at the
Shigeki Sato et al.
Chemical communications (Cambridge, England), (41), 6264-6266 (2009-10-15)
A versatile, highly enantiocontrolled entry to the spiro-beta-lactam core of chartellines has been developed by expanding the scope of oxidative nitrogen atom transfer methodology based on chiral Rh-nitrenoid species.
Liangbing Fu et al.
Organic letters, 20(8), 2399-2402 (2018-04-12)
Rhodium(II)-catalyzed reactions between isopropyl acetate and trichloroethyl aryldiazoacetates result in the formation of oxirane intermediates that ring open under the reaction conditions to form tertiary alcohols. When the reaction is catalyzed by the dirhodium tetrakis(triarylcyclopropanecarboxylate) complex, Rh2( S-2-Cl,4-BrTPCP)4, the tertiary
Hengbin Wang et al.
Chemical science, 4(7), 2844-2850 (2013-09-21)
The rhodium-catalyzed reaction of electron-deficient alkenes with substituted aryldiazoacetates and vinyldiazoacetates results in highly stereoselective cyclopropanations. With adamantylglycine derived catalyst Rh2(S-TCPTAD)4, high asymmetric induction (up to 98% ee) can be obtained with a range of substrates. Computational studies suggest that
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.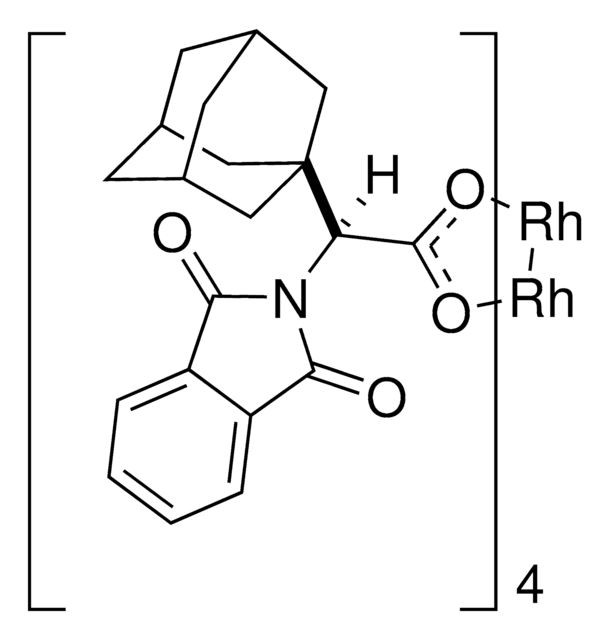



![Bis[rhodium(α,α,α′,α′-tetramethyl-1,3-benzenedipropionic acid)] 95%](/deepweb/assets/sigmaaldrich/product/structures/102/178/d1171a49-0358-406b-8b32-04324dbf9c02/640/d1171a49-0358-406b-8b32-04324dbf9c02.png)