904988
Feng L3-PiPr2
Sinonimo/i:
(2S,2′S)-1,1′-(propane-1,3-diyl)bis(2-((2,6-diisopropylphenyl)carbamoyl)piperidine-1-oxide)
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C39H60N4O4
Numero CAS:
Peso molecolare:
648.92
Codice UNSPSC:
12352300
Prodotti consigliati
Forma fisica
solid
Temperatura di conservazione
2-8°C
Applicazioni
L3-PiPr2 is a chiral N,N-dioxide ligand developed by the Feng group. In conjunction with a variety of metal salts, this versatile ligand forms and active catalysts complex with application in many different reactions.
Altre note
An N,N′-Dioxide/In(OTf)3 Catalyst for the Asymmetric Hetero-Diels–Alder Reaction Between Danishefsky′s Dienes and Aldehydes: Application in the Total Synthesis of Triketide
Enantioselective Allylation of Ketones Catalyzed by N,N′-Dioxide and Indium(III) Complex
Asymmetric Dearomatization of Indoles through a Michael/Friedel-Crafts-Type Cascade To Construct Polycyclic Spiroindolines
Enantioselective Allylation of Ketones Catalyzed by N,N′-Dioxide and Indium(III) Complex
Asymmetric Dearomatization of Indoles through a Michael/Friedel-Crafts-Type Cascade To Construct Polycyclic Spiroindolines
Prodotti correlati
N° Catalogo
Descrizione
Determinazione del prezzo
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Lot/Batch Number
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
An N,N'-dioxide/In(OTf)3 catalyst for the asymmetric Hetero-Diels-Alder reaction between Danishefsky's dienes and aldehydes: application in the total synthesis of triketide.
Zhipeng Yu et al.
Angewandte Chemie (International ed. in English), 47(7), 1308-1311 (2008-01-08)
Hang Zhang et al.
Chemical communications (Cambridge, England), 54(88), 12511-12514 (2018-10-23)
The catalytic asymmetric ene-type reactions of vinylogous hydrazone were accomplished by using chiral N,N'-dioxide-metal salt complexes as catalysts. A wide range of electrophiles, including isatins, α-ketoester, imines, and aldehydes reacted with (E)-2-methyl-N-(piperidin-1-yl)prop-2-en-1-imine efficiently, affording the corresponding homoallylic alcohols and amines
Xin Zhang et al.
The Journal of organic chemistry, 72(14), 5227-5233 (2007-06-15)
Complexes of (S)-pipecolic acid-, L-proline-, and other amino acid-derived N,N'-dioxides coordinated with different metal ions have been investigated in the enantioselective allylation of ketones. A variety of aromatic ketones were found to be suitable substrates in the presence of the
Xiaohu Zhao et al.
Angewandte Chemie (International ed. in English), 54(13), 4032-4035 (2015-02-05)
A highly efficient asymmetric dearomatization of indoles was realized through a cascade reaction between 2-isocyanoethylindole and alkylidene malonates catalyzed by a chiral N,N'-dioxide/Mg(II) catalyst. Fused polycyclic indolines containing three stereocenters were afforded in good yields with excellent diastereo- and enantioselectivities
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.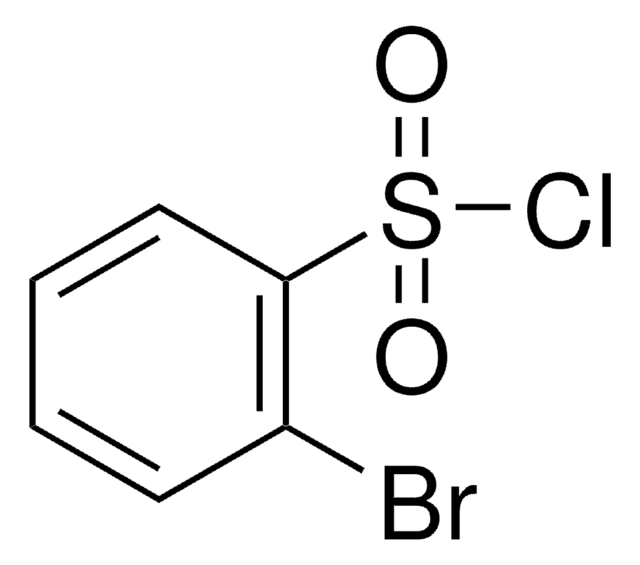
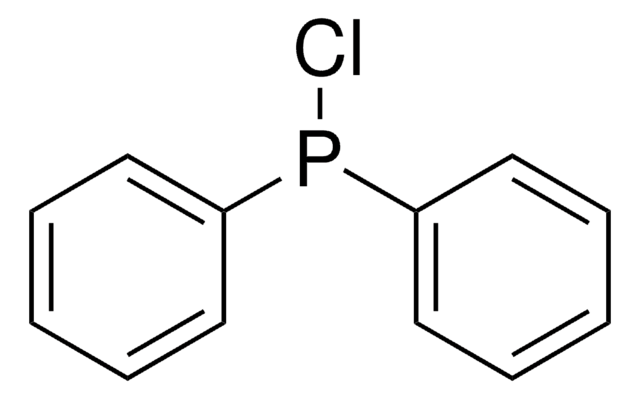
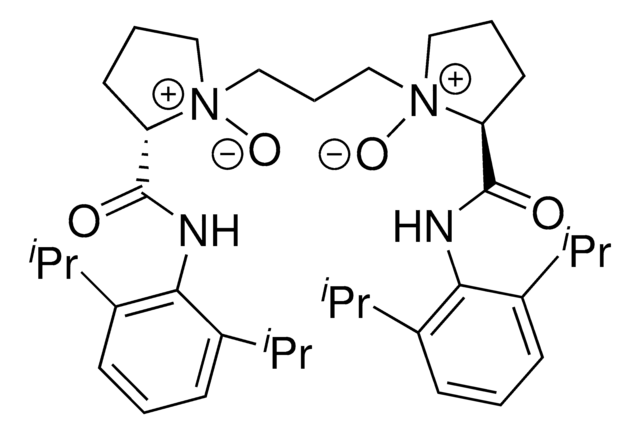
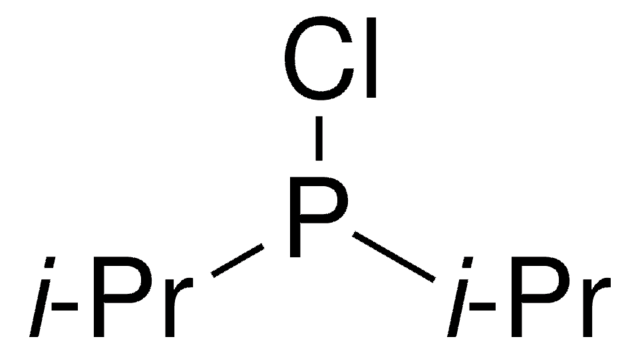
![N-[(1R,2R)-2-(1-Piperidinyl)cyclohexyl]-N′-[4-(trifluoromethyl)phenyl]squaramide 95%](/deepweb/assets/sigmaaldrich/product/structures/238/480/7149c9c0-8769-418a-a96c-77c15dd50cd0/640/7149c9c0-8769-418a-a96c-77c15dd50cd0.png)