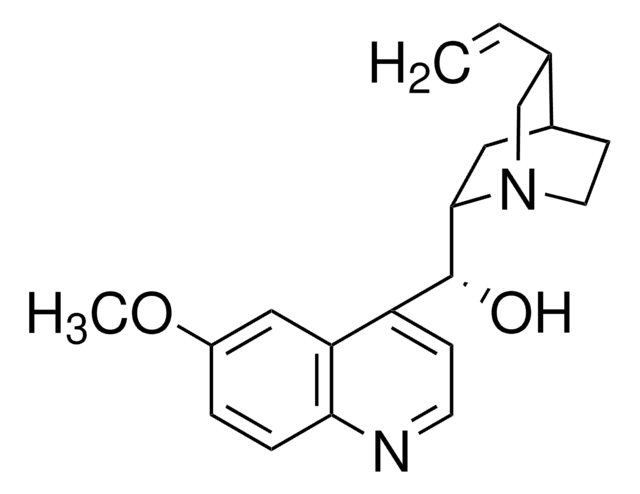
690481
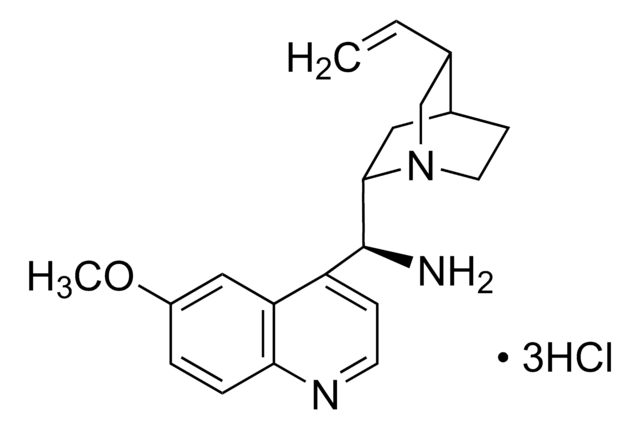
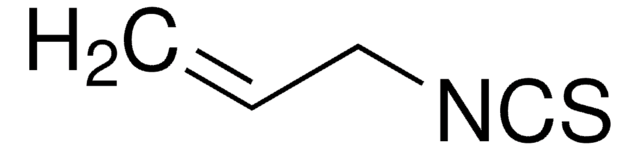
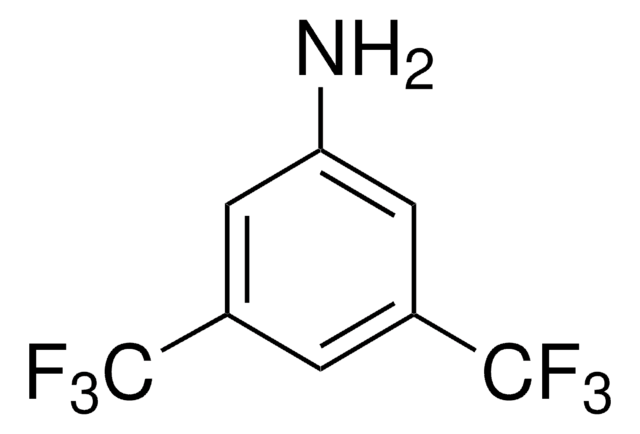
N-[3,5-Bis(trifluoromethyl)phenyl]-N′-[(8a,9S)-6′-methoxy-9-cinchonanyl]thiourea
90%
Sinonimo/i:
1-[3,5-Bis(trifluoromethyl)phenyl)-3-{(S)(6-methoxy-4-quinolinyl)-[(2S,4S,5R)-5-vinyl-1-aza-bicyclo[2.2.2]oct-2-yl]methyl}thiourea, epi-N-Quinidyl-N′-bis(3,5-trifluoromethyl)phenylthiourea
About This Item
Prodotti consigliati
Saggio
≥89.0%
90%
Stato
lumps
Gruppo funzionale
amine
fluoro
thiourea
Stringa SMILE
COc1ccc2nccc([C@H](NC(=S)Nc3cc(cc(c3)C(F)(F)F)C(F)(F)F)C4CC5CCN4C[C@@H]5C=C)c2c1
InChI
1S/C29H28F6N4OS/c1-3-16-15-39-9-7-17(16)10-25(39)26(22-6-8-36-24-5-4-21(40-2)14-23(22)24)38-27(41)37-20-12-18(28(30,31)32)11-19(13-20)29(33,34)35/h3-6,8,11-14,16-17,25-26H,1,7,9-10,15H2,2H3,(H2,37,38,41)/t16-,17-,25-,26-/m0/s1
IQMKPBFOEWWDIQ-FRSFCCSCSA-N
Categorie correlate
Applicazioni
- Stereoselective diaryl(nitro)butanone via enantioselective Michael addition of nitromethane to chalcones.
- Enantioselective β-amino acids via asymmetric Mannich reaction of malonates with aryl and alkyl imines.
- The synthesis of 3-indolylmethanamines by the reaction of indoles with imines via asymmetric Friedel-Crafts reaction.
- The enantioselective conjugate addition of active methylene compounds to enones to obtain the corresponding addition products.
Confezionamento
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Oral
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.![1-[3,5-bis(trifluoromethyl)phenyl]-3-[(1R,2R)-(-)-2-(dimethylamino)cyclohexyl]thiourea AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/236/021/d944889d-2233-4700-9f2c-caa3652d0124/640/d944889d-2233-4700-9f2c-caa3652d0124.png)
![N-[(1R,2R)-2-(1-Piperidinyl)cyclohexyl]-N′-[4-(trifluoromethyl)phenyl]squaramide 95%](/deepweb/assets/sigmaaldrich/product/structures/238/480/7149c9c0-8769-418a-a96c-77c15dd50cd0/640/7149c9c0-8769-418a-a96c-77c15dd50cd0.png)
![N-[3,5-Bis(trifluoromethyl)phenyl]-N′-[(8a,9S)-10,11-dihydro-6′-methoxy-9-cinchonanyl]thiourea 90%](/deepweb/assets/sigmaaldrich/product/structures/321/413/b3c0b73b-9ee7-4dea-b9cd-795beb4cdbfa/640/b3c0b73b-9ee7-4dea-b9cd-795beb4cdbfa.png)
![1-[3,5-Bis(trifluoromethyl)phenyl]-3-[(1R,2R)-(−)-2-(dimethylamino)cyclohexyl]thiourea](/deepweb/assets/sigmaaldrich/product/structures/384/772/d336462c-f438-446d-be0c-4064705213cc/640/d336462c-f438-446d-be0c-4064705213cc.png)
![(S)-2-[[3,5-Bis(trifluoromethyl)phenyl]thioureido]-N-benzyl-N-3,3-trimethylbutanamide 97%](/deepweb/assets/sigmaaldrich/product/structures/373/888/118b46f2-6c2e-4a87-8266-c4dbcd5db51f/640/118b46f2-6c2e-4a87-8266-c4dbcd5db51f.png)